Engineering the orbital character of the electronic structure of superconducting cuprates
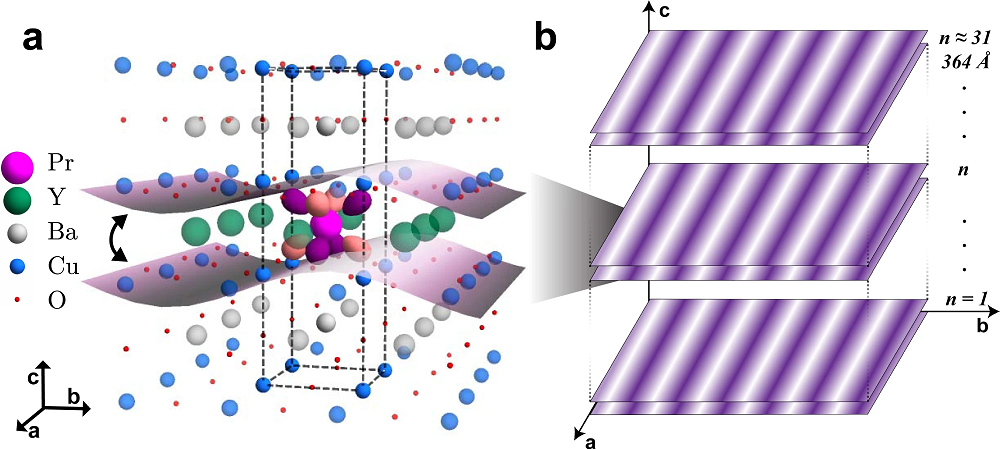
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K. This family is known as cuprates as it can be viewed as containing anionic copper complexes. Many YBCO compounds have the general formula YBa2Cu3O6+δ. The shape of 3d-orbitals often governs the electronic and magnetic properties of correlated transition metal oxides. In the superconducting cuprates, the planar confinement of the dx2-y2 orbital dictates the two-dimensional nature of the unconventional superconductivity and a competing charge order.
The cuprate phase diagram illustrates a quintessential example of a low-dimensional correlated quantum system: a multitude of fascinating electronic phases, including charge order and high-temperature superconductivity, emanating from the combination of complex interactions that govern a simple two-dimensional chemical structure, the CuO2 planes. These interactions within the CuO2 planes are largely influenced by the underlying characteristics of the anisotropic, planar Cu 3dx2-y2 orbitals
Charge ordering (CO) is a phase transition occurring mostly in strongly correlated materials such as transition metal oxides or organic conductors. Due to the strong interaction between electrons, charges are localized on different sites, leading to a disproportionation and an ordered superlattice. The charge order transition is often is closely interconnected with superconductivity.
In (YBCO), the highest reported out-of-plane correlation length is nearly an order of magnitude smaller than the highest reported in-plane correlation length, highlighting the two-dimensional nature of the CO phase. However, it has been observed that the application of certain perturbations — high magnetic fields, epitaxial strain in thin films, or uniaxial strain— can induce a second CO peak, evidencing an out-of-plane coupling that locks the phase of adjacent CuO2 planes. While it is easy to discern the two-dimensional nature of the unperturbed CO due to the underlying planar 3dx2-y2 orbitals, the mechanisms by which these external perturbations are able to induce a three-dimensional CO peak remain unclear.
The experimental study of this phenomenon presents complicated technical challenges that preclude many techniques altogether, making it difficult to systematically investigate how the dimensionality of the CO can be tuned and obscuring its connection with superconductivity. Achieving orbital-specific control of the electronic structure to allow coupling pathways across adjacent planes would enable direct assessment of the role of dimensionality in the intertwined orders. In this vein, a team of researchers has now hypothesized 1 that three-dimensional CO could be stabilized by virtue of tuning the underlying orbital character via hybridization to more directly enhance the out-of-plane coupling between adjacent CuO2 plane layers.
The researchers chose praseodymium (Pr), which is the largest trivalent rare-earth ion that can form a YBCO structure, to substitute some yttrium atoms in YBa2Cu3O6+δ because hybridization would emerge between the Pr 4f orbitals and planar CuO2 states, yielding an electronically relevant, hybridized orbital with spatial extension in three dimensions. This results in PrxY1−xBa2Cu3O7 .
Superconductivity gains additional three-dimensional character with increasing Pr substitution and has been attributed to increased coupling between CuO2 planes through the bonding with the substituted Pr ions. Various results suggest that localized Pr 4f states are appreciably hybridized with the valence band states associated with the conducting CuO2 planes. The researchers show through density-functional calculations that, through this hybridization which is unique to Pr, the CO on adjacent CuO2 planes can couple to yield a stable three-dimensional CO phase.
Altogether, these results constitute the first detection of a fully stabilized, long-range three-dimensional CO that competes with superconductivity, achieved by intrinsically engineering the orbital character of the electronic structure. They demonstrate how the influence of underlying orbital character on an electronic phase can be tuned via orbital hybridization, something that can be generalized to other correlated transition metal oxides and layered systems.
More on the subject:
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers
References
- Alejandro Ruiz, Brandon Gunn,Yi Lu, Kalyan Sasmal, Camilla M. Moir, Rourav Basak, Hai Huang, Jun-Sik Lee, Fanny Rodolakis, Timothy J. Boyle, Morgan Walker, Yu He, Santiago Blanco-Canosa, Eduardo H. da Silva Neto, M. Brian Maple & Alex Frano (2022) Stabilization of three-dimensional charge order through interplanar orbital hybridization in PrxY1−xBa2Cu3O6+δ. Nat Commun doi: 10.1038/s41467-022-33607-z ↩