Crosslinking pectin for the simultaneous removal of multiple pollutants from water
Crosslinking pectin for the simultaneous removal of multiple pollutants from water
Chemical contamination of water bodies on one hand, and water shortages due to overexploitation on the other, have increased the need for effective and efficient water treatment and decontamination processes. Two important aspects need to be taken into consideration to define what is an “effective and efficient” treatment. First, as current methods of removing pathogens, organic impurities, and salts from water require large amounts of energy, new processes need to be affordable and conserve energy. Then, as new contaminants continuously appear in water bodies because of the generation of new chemicals at a high rate, new methods need to be flexible.
Adsorption is a promising technique for decontaminating water and has been used for a long time. Compared with other methods, it is relatively inexpensive and can be implemented in water treatment plants or domestic treatments without excessive energy consumption. Adsorbents can be environmentally friendly if the materials used in their fabrication are biodegradable and do not contain nanoparticles. The development and implementation of ecologically sustainable adsorption technologies can help to overcome the challenges of water pollution and water scarcity in a cost-effective and energy-efficient manner.
Pectin, a polysaccharide present in the cellular walls of plants, has been used to remediate water against mono-, di-, and trivalent heavy metals (such as Zn2+, Ni2+, and Cd2+) with high efficiency and the possibility of reusing the adsorbent. Pectin-based adsorbents must be insoluble for use in water. In order to achieve this, crosslinking with calcium ions is quite, although other metal ions have also been used.
Divalent metal ions, such as Ca2+, promote the gelation of pectin. The crosslinks are established between the carboxylate groups (COO−, i.e., non-methyl-esterified residues) of neighboring pectin chains via the Ca2+ ions, which replace the hydrogen bonds in neat pectin. The degree of crosslinking in pectin is related to its degree of methoxylation (DM), which represents the proportion of methyl-esterified carboxyl groups (COOCH3) in comparison to non-esterified carboxyl groups (COOH). Pectin with a low DM demonstrates a more efficient crosslinking behaviour. In addition to Ca2+, pectin can also be reticulated by cations such as Zn2+. Crosslinking with Zn2+ results in a more heterogeneous network structure because it interacts with both carboxyl and hydroxyl groups, whereas Ca2+ interacts only with carboxyl groups.
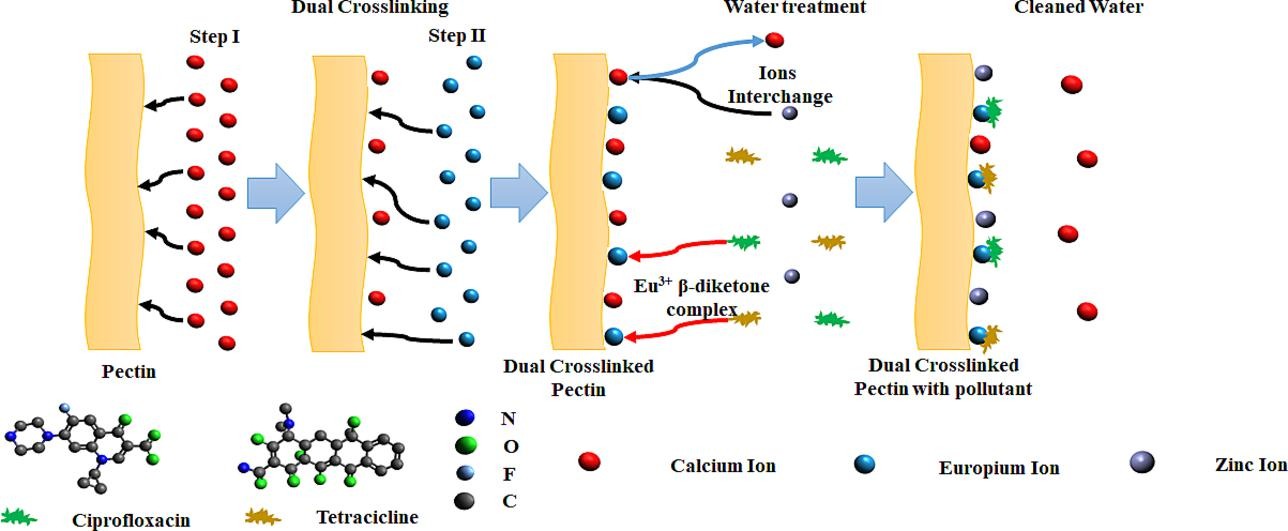
The main limitation of pectin-based adsorbents is that they are useful only for the removal of heavy metals and no other contaminants, such as pharmaceuticals and other emerging pollutants. To address this, a team of researchers has now used 1 europium (Eu) to crosslink pectin to enhance the removal of pharmaceutical products.
Eu is a lanthanide that reacts with different pharmaceuticals. It has been used as a fluorescent probe to detect hydrochlorothiazide (a diuretic drug) and tetracycline (an antibiotic). The interactions between pharmaceuticals and Eu can also be used for other purposes. It is also important to note that the acute toxicity of Eu. Eu was classified as a “category 4″ material, which means that Eu has low toxicity when used in deficient concentrations, as those employed in this work.
The researchers developed a novel approach to enhance the remediation capacity of pectin (with a low degree of methylation) by crosslinking it with different agents: calcium, europium, and their combination. They performed scanning electron microscopy, infrared spectroscopy, and X-ray diffraction experiments to understand the molecular structure of pectin after gelation with the three agents. The results showed that calcium, europium, and their combination all induce the gelation of pectin.
However, the reticulated pectin structures exhibited significant structural differences depending on the type of crosslinking agent used, which affected the adsorption capacity. Specifically, calcium cations partially formed a crystalline structure, whereas europium cations produced a more homogeneous network without crystalline regions. The dual-crosslinking system comprising calcium and europium cations resulted in an intermediate network with both crystalline and amorphous regions.
These findings suggest that dual-cross-linked pectin is a highly effective adsorbent for the simultaneous removal of both heavy metals and pharmaceutical products. This novel approach of crosslinking pectin with multiple agents has the potential to significantly enhance its remediation capacity, offering a promising solution for the simultaneous removal of multiple pollutants from water.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Javier Martínez-Sabando, Francesco Coin, Juan Carlos Raposo, Aitor Larrañaga, Jorge H. Melillo, Silvina Cerveny (2023) Dual crosslinking of low-methoxyl pectin by calcium and europium for the simultaneous removal of pharmaceuticals and divalent heavy metals Chemical Engineering Journal doi: 10.1016/j.cej.2023.146162 ↩