A new recycling approach to complex plastic waste
A new recycling approach to complex plastic waste
Author: Izotz Amundarain, Researcher in Recycling and Circular Economy at GAIKER Technology Centre, Basque Research and Technology Alliance (BRTA)
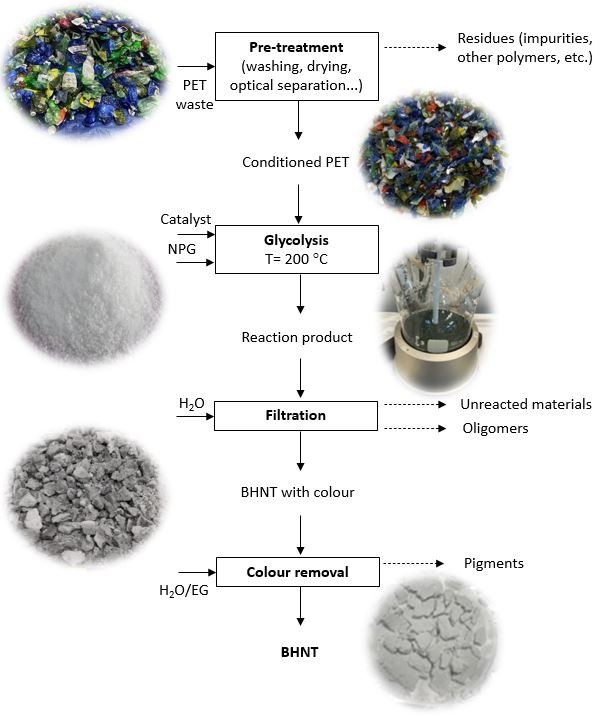
PET (polyethylene terephthalate), a widely used polymer in packaging due to its favourable properties, increases rapidly in global plastic production and waste. Between 2000 and 2019, global plastic production rose from 234 to 460 million tonnes and is projected to reach 1,231 million tonnes by 2060. As a result, plastic waste more than doubled from 156 to 353 million tonnes in the same period, with only 9% being recycled in 2019. PET is one of the most recycled plastics in Europe, with a 60% collection rate in 2022, mainly in the form of bottles. However, much of the remaining PET waste is incinerated or landfilled. Mechanical recycling, the most common method, struggles with complex waste streams. Therefore, chemical recycling methods like glycolysis are being explored, which break down PET into monomers using glycols. Ethylene glycol is commonly used, with zinc acetate as an effective catalyst. A new study explores the use of neopentyl glycol (NPG), a less-studied solvent, which produces more chemically resistant and thermally stable monomers. A purification step was also developed to remove dyes and impurities, enabling the transformation of complex PET waste into high-value monomers for new product manufacturing.
Researchers from the GAIKER Technology Centre and the University of the Basque Country (UPV/EHU) explored 1 the use of NPG as a novel solvent for the chemical recycling of complex PET waste. NPG is commonly used in the production of resins, paints, and coatings due to its stability and resistance to heat, light, and water.
Researchers carried out chemical recycling of PET plastic waste using a process called glycolysis. They used a special type of alcohol, NPG, and zinc acetate as a catalyst to break down the plastic. The reaction took place in a glass reactor heated to 200 °C, with PET and NPG mixed in a 6:1 molar ratio. They tested the process at different times (1–4 hours) and temperatures (180–220 °C) to see how well the plastic converted into useful products. The goal was to produce BHNT, a novel monomer that could be used in resins and coatings.
PET conversion was nearly complete in 2 hours for most samples, except for PET from EoL tyres containing rubber and other textile fibres, causing lower conversion. Extending the reaction beyond equilibrium led to a shift from depolymerization to polymerization, increasing the dimer content.
After the reaction, the mixture was filtered and treated with hot water to remove unwanted parts. The raw BHNT was then crystallized and filtered to isolate the product. Since the raw BHNT still contained color and metal impurities from the original PET waste, it was further purified using water, ethylene glycol, and activated carbon to remove color and reduce metal content, especially zinc. The purification process successfully increased the BHNT content to over 90%, with minimal dimer and asymmetric species. Additionally, the purification reduced the color intensity and zinc content, improving the quality of the final product. The purified BHNT showed a molecular weight close to the theoretical value, indicating successful glycolysis and purification.
The purified BHNT was analyzed using various instruments to study its chemical structure, thermal properties, and color. Techniques included FTIR, DSC, colorimetry, ¹H-NMR, and ICP-OES. These tests confirmed the effectiveness of the recycling and purification process.
The study shows that NPG, a new chemical used in recycling, can break down different types of PET plastic waste—like colored bottles, multilayer packaging, and textiles—into useful building blocks called BHNT monomers. The best results happened when using a 6:1 ratio of NPG to PET at 200 °C, achieving up to 95.6% PET breakdown and 87.4% yield of the monomer in just 2 hours.
However, when the PET waste also contained other materials like PE, cotton, or rubber, the monomer yield dropped by about 20%. To fix this, the researchers created a special cleaning step to remove dyes and impurities, which increased the purity of the monomers to over 90%.
Overall, the method looks very promising for recycling complex PET waste that can’t be recycled by mechanical means. It can help reduce environmental harm and support a circular economy. The next step is to scale up this method using a pilot plant of 140 L.
The BRTA is a consortium that remains a step ahead of future socio-economic challenges worldwide and in the Basque Autonomous Community; it addresses them through research and technological development, thus projecting itself internationally. The BRTA centres collaborate to generate knowledge and transfer it to Basque society and industry so as to make them more innovative and competitive. The BRTA is an alliance of 17 R&D centres and cooperative research centres with the support of the Basque Government, the SPRI and the Chartered Provincial Councils of Araba, Bizkaia and Gipuzkoa.
References
- Amundarain, I; López-Montenegro, S; Asueta, A; Arnaiz, S; Pereda-Ayo, B. (2025) Neopentyl glycol as alternative solvent for the chemical recycling of complex PET waste Materials Advances doi: 10.1039/D4MA00919C ↩
