Targeting aberrant DNA methylation in mesenchymal stromal cells as a treatment for myeloma bone disease
Targeting aberrant DNA methylation in mesenchymal stromal cells as a treatment for myeloma bone disease
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Multiple myeloma (MM) is an incurable hematological malignancy characterized by clonal expansion of plasma cells in the bone marrow (BM) 1. Nearly 90% of myeloma patients suffer from skeletal-related events during the course of the disease that not only affect the quality of life but also their overall survival 2. Myeloma-associated bone disease (MBD) is characterized by an increase in bone-resorptive activity and number of osteoclasts (OCs), as well as impairment of bone-forming activity and differentiation of osteoblasts (OBs), which ultimately lead to the development of osteolytic lesions 3. In this regard, it has been widely shown that a complex and bidirectional relationship exists between MM cells and the BM niche, which results in an uncoupling of the bone remodeling process4.
Mesenchymal stromal cells (MSCs) are an essential cell type in the formation and function of the BM microenvironment, being the progenitors of bone-forming OBs, adipocytes, and chondroblasts, as well as the hematopoietic-supporting stroma components of the BM 5. It is well-documented that BM-derived MSCs from MM patients contribute to MM progression (4) and show an impaired ability to differentiate into OBs 6.
Furthermore, bone lesions persist in many MM patients even after therapeutic remission, suggesting a long-term defect in MSCs that inhibit their ability to properly differentiate into functional OBs 7. Previous studies described that MSCs from MM patients are cytogenetically normal 8, but show alterations in their transcriptional (6) and proteomic (4) profiles even in the absence of myeloma cell interaction. This suggests that epigenetic mechanisms could be governing the tumor-promoting functions of MSCs and their prolonged OB suppression in MM. However, there is a lack of information about DNA methylation-related mechanisms that may contribute to MM progression and subsequent bone defects. Thus, in the present work, García-Gómez A and collaborators aimed to identify these DNA methylation alterations in MSCs of MM patients 9.
Firstly, authors obtained genome-wide DNA methylation profiles of BM-derived MSCs isolated at different stages of MM and healthy controls. They identified DNA methylation changes using two different statistical approaches: (I) detection of differentially methylated CpG positions (DMPs) between patient and healthy MSCs; and (ii) detection of differentially variable CpG positions (DVPs) between the sample groups. Thus, they observed stochastic and heterogeneous DNA methylation patterns which are associated with early stages of carcinogenesis, and that the majority of identified DMPs and DVPs are disease stage-specific. Given that myeloma is a multi-stage disease, they then analyzed the accumulative changes of DNA methylation associated with MM progression. Thus, they performed a search for enriched transcription factor (TF)-binding sites in these regions and observed that DMP sites which experienced aberrant DNA hypomethylation were highly enriched in binding motifs of the Homeobox family, and, interestingly, genes of this family are involved in the regulation of bone formation 10.
To further investigate the relationship between differential DNA methylation and gene expression, they mapped the DMPs to the most proximal gene. Using expression array data from BM-derived MSCs of healthy controls and MM patients, they observed that the genes displaying both differential methylation and expression were enriched in functional categories important in cell fate commitment and bone phenotype. Specifically, hypermethylated genes that showed a reduced expression in patient MSCs include positive regulators of OB differentiation. In contrast, negative regulators of osteogenesis were hypomethylated and consequently upregulated in patient MSCs. Besides, upon a closer inspection of several Homeobox-associated genomic regions, they observed a negative correlation between DNA methylation of promoters and gene expression. In fact, genes of this family have been reported as regulators of bone creation and showed an association between DNA methylation at gene promoter and gene expression.
To address the potential contribution of MM cells in mediating aberrant DNA methylation changes in MSCs, they evaluated whether the epigenetic changes observed in MM-MSCs could be mimicked in vitro by direct contact of healthy MSCs with MM cells. Thus, they co-cultured BM-derived MSCs from healthy donors with a human MM cell line. Under these conditions, they validated the inhibitory effect of MM cells in MSC-to-OB differentiation and observed a decrease in both alkaline phosphatase activity (ALP), which indicates the formation of new bone, and OB mineralization in OBs differentiated in the presence of conditioned media from the MM cell line as compared to OBs differentiated alone. They then investigated the DNA methylation profiling of MSCs from healthy donors generated upon interaction with MM cells. Similar to that observed in primary patient MSCs, gene analyses revealed enrichment in Homeobox genes and categories related to bone formation.
Gene expression analysis of DNA methyltransferases (DNMTs) in MSCs from MM patients co-cultured with MM cells obtained from a previous study showed an aberrant upregulation of DNMT1. DNMT1 interacts with the methyltransferase G9a to coordinate DNA methylation during cell replication promoting transcriptional silencing of target genes 11.
In this regard, authors hypothesized that the dual inhibition of DNMT1 and G9a could reactivate hypermethylated and silenced genes of MSCs from MM patients preserving their osteogenic potential and therefore preventing myeloma-associated bone loss. Thus, they utilized CM-272, a dual inhibitor of DNMTs and G9a, and observed that was able to restore the expression of Homeobox genes that were epigenetically repressed in MSCs from MM patients. Mechanistically, they observed a loss of DNA methylation in the promoter region of the majority of the aforementioned genes after CM-272 treatment in MM-MSCs. Next, they addressed whether targeting DNMT and G9a may have a role in regulating osteogenic differentiation. For this purpose, they cultured MSCs from myeloma patients in osteogenic media to obtain differentiated OBs in the presence or absence of CM-272. Interestingly, CM-272 was able to increase ALP activity in early-stage OBs and to upregulate the relative expression of several late bone formation markers in MSCs from myeloma patients. Previous research has described that MM cells exert their effect on MSCs through both direct cell-cell contact and soluble factor mechanisms (4).
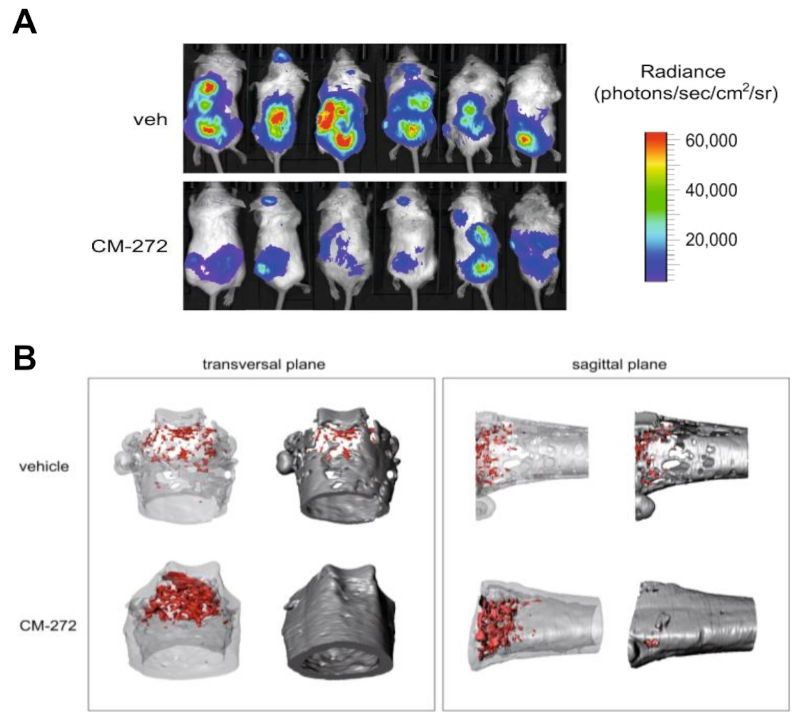
For this purpose, utilizing a transwell system avoiding contact between both cell types, they observed that soluble factor secreted by MM cell lines were sufficient to change the expression of several OB-relevant genes in healthy MSCs, concordantly to direct co-culture. Furthermore, treatment with the dual inhibitor CM-272 was able to partially reverse those changes in gene expression mediated by soluble factors secreted by MM cells. To test the effect of CM-272 in the context of MBD, they used an established murine model of bone marrow-disseminated myeloma. After engraftment of myeloma cells, mice were treated for 4 weeks with CM-272. Interestingly, CM-272 controlled tumor progression and preserved trabecular structures in distal femurs. Besides, when they further examine the in vivo effect of CM-272 on DNA methylation of myeloma-associated MSCs, they observed that myeloma-bearing mice that were treated with CM-272 displayed a partial reversion of aberrant hypermethylation of MSCs caused by the presence of myeloma cells. As previously described, these DNA methylation changes occurred at genomic loci enriched for genes from the Homeobox family. And importantly, CM-272 treatment was able to restore the DNA methylation levels at these loci to resemble that of healthy mice.
Altogether, these results demonstrate that epigenetic aberrancies mediate the impairment of bone formation in MM, and its targeting by CM-272 is able to reverse MBD.
References
- San-Miguel JF, Kantarjian HM. Multiple myeloma and chronic leukaemias in 2014: improved understanding of disease biology and treatment. Nat Rev Clin Oncol. 2015; 12: 71-2. PMID: 25511190 DOI : 10.1038/nrclinonc.2014.216. ↩
- Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease Cancer. 2007 Oct; 110 :1860-7. PMID: 17763372 DOI: 10.1002/cncr.22991. ↩
- Panaroni C, Yee AJ, Raje NS. Myeloma and Bone Disease. Curr Osteoporos Rep. 2017; 15 :483-98. PMID: 28861842 DOI: 10.1007/s11914-017-0397-5. ↩
- Garcia-Gomez A, Sanchez-Guijo F, Del Cañizo MC, San Miguel JF, Garayoa M. Multiple myeloma mesenchymal stromal cells: contribution to myeloma bone disease and therapeutics. World J. Stem Cells. 2014; 6: 322-43. PMID: 25126382 DOI: 10.4252/wjsc.v6.i3.322. ↩
- Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I. & Frolova, G. P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6: 230-47. PMID: 5654088. ↩
- Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, Danho C, Laharrague P, Klein B et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007; 21: 1079-88. PMID: 17344918 DOI: 10.1038/sj.leu.2404621. ↩
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016; 91: 719-34. PMID: 27291302 DOI: 10.1002/ajh.24402. ↩
- Garayoa M, Garcia JL, Santamaria C, Garcia-Gomez A, Blanco JF, Pandiella A, Hernández JM, Sanchez-Guijo FM et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009; 23: 1515-27 (2009). PMID: 19357701 DOI: 10.1038/leu.2009.65. ↩
- Garcia-Gomez A, Li T, de la Calle-Fabregat C, Rodríguez-Ubreva J, Ciudad L, Català-Moll F, Godoy-Tena G, Martín-Sánchez M et al. Targeting aberrant DNA methylation in mesenchymal stromal cells as a treatment for myeloma bone disease. Nat Commun. 2021; 12: 421. PMID: 33462210 DOI: 10.1038/s41467-020-20715-x. ↩
- Wei XF, Chen QL, Fu Y, Zhang QK. J. Wnt and BMP signaling pathways co-operatively induce the differentiation of multiple myeloma mesenchymal stem cells into osteoblasts by upregulating EMX2. Cell Biochem. 2019; 120: 6515-27. PMID: 30450775 DOI: 10.1002/jcb.27942. ↩
- Estève PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, Carey MF, Pradhan S. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 2006; 20:3089-103. PMID: 17085482 doi: 10.1101/gad.1463706. ↩