Targeting WDR5/ATAD2 signaling by the CK2/IKAROS axis demonstrates therapeutic efficacy in T-ALL
Targeting WDR5/ATAD2 signaling by the CK2/IKAROS axis demonstrates therapeutic efficacy in T-ALL
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological malignancy with a poor prognosis and limited options for targeted therapies 1. Since the targeted therapies benefiting T-ALL are still limited owing to the biological heterogeneity of T-ALL 2, identifying novel “druggable” molecular markers and illustrating the underlying mechanisms are immediate pressing issues in T-ALL 3. To date, epigenome-targeting therapies have shown significant therapeutic benefits in various hematological malignancies 4. However, no such treatments are currently available for T-cell acute lymphoblastic leukemia (T-ALL). In a recent work 5, Han Q and coworkers aimed to analyze this approach in more depth and identified a novel epigenomic targeting strategy in T-ALL by inhibiting the essential histone modifier WD repeat-containing protein 5 (WDR5) providing a rationale for clinical evaluation of epigenome-targeting approaches in T-ALL therapy.
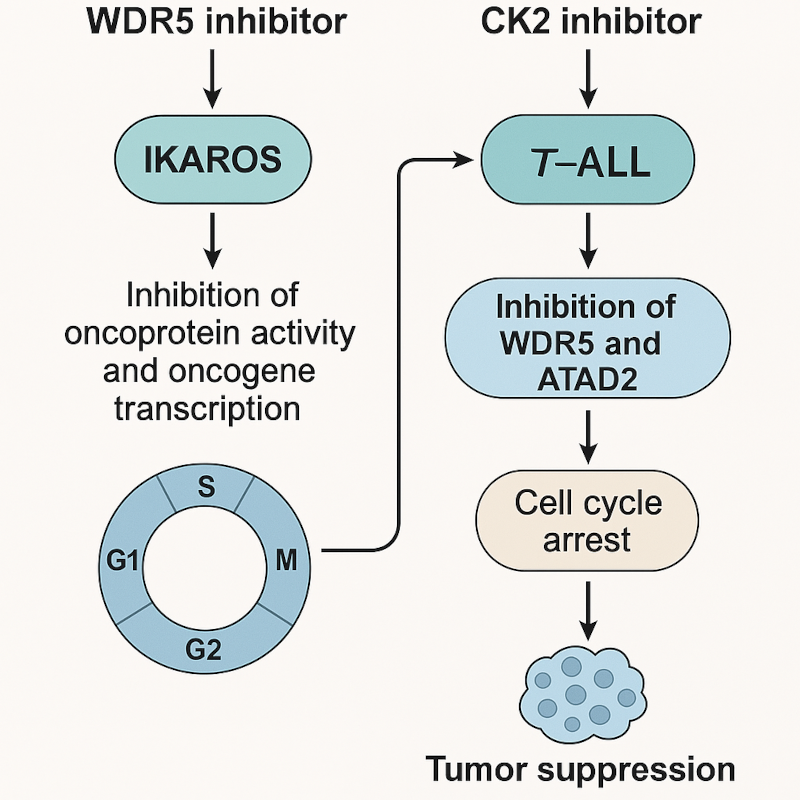
Image adapted from Han Q et al. Blood (2025) DOI: 10.1182/blood.2024024130.
Taking this data into account, authors firstly analyzed the genetic expression of T-ALL cohorts compared to healthy controls and observed that WDR5 was significantly overexpressed in T-ALL and associated with poorer survival, higher relapse rate and more high-risk patients. Moreover, stratifying patients into WDR5-high and WDR5-low groups based on WDR5 mRNA expression levels in T-ALL cohorts revealed a significant enrichment of cell cycle–related genes and activation of cell cycle signaling pathways in the WDR5-high group, underscoring the oncogenic role of WDR5. To examine in greater detail its oncogenic role, they genetically silenced WDR5 using WDR5 shRNA in leukemic T-ALL cells lines. As expected, WDR5 knockdown (KD) significantly inhibited cell proliferation, induced the G1 phase arrest and downregulated the G1/S phase transition compared with control cells. Then, WDR5 silenced and control cells were respectively injected into immunodeficient mice and results showed that WDR5 silencing significantly reduced the spleen size, spleen weight, and the percentage of leukemic cells in the spleen and bone marrow (BM). Similarly, they observed that OICR-9429, a small molecule inhibitor of WDR5 6, successfully inhibited T-ALL cell proliferation. Furthermore, the drug provoked the cell cycle arrest in the G1 phase in leukemic cells. Interestingly, whole-transcriptome analysis comparing healthy cells and leukemic cells following OICR-9429 treatment revealed a significant enrichment of cell cycle-related genes among the differentially expressed transcripts, highlighting the compound’s impact on cell cycle regulation.
Next, authors aimed to evaluate the signaling pathway involved in WDR5 activation. Casein kinase II (CK2) is a ubiquitous and highly conserved eukaryotic serine/threonine-protein kinase which plays a vital role in cell cycle progression, cell differentiation, and transcription regulation 7. On the other hand, IKAROS is a direct target of CK2 and importantly, a potent tumor suppressor in ALL cancer cells, which, interestingly, is overexpressed in T-ALL 8. Previous works highlighted that CX-4945, a CK2 inhibitor, was effective suppressing WDR5 by restoring the IKAROS function in B-ALL 9. Similar to observations in B-ALL, CX-4945 also suppressed WDR5 expression in T-ALL by restoring IKAROS function. Besides, they observed that silencing of IKAROS-encoding gene IKZF1 significantly increased WDR5 expression whereas IKAROS overexpression reduced WDR5. Importantly, IKAROS expression was able to represses the activity of the WDR5 promoter. Then, to analyze the functional implication of WDR5 silencing in T-ALL engraftment, they injected leukemic control cells and leukemic cells devoided of WDR5 into immunodeficient mice. Next, mice from each engraftment type were divided into 2 cohorts and treated with either vehicle or CX-4945. Interestingly, mice injected with WDR5-deficient leukemic cells and treated with CX-4945 exhibited significantly prolonged survival, reduced spleen size and weight, and a lower percentage of human T-ALL cells in both the spleen and BM, compared to mice receiving either CX-4945-treated control leukemic cells or untreated WDR5-silenced cells.
After observing these results, the authors evaluated the possibility of a synergistic effect of CX-4945 with the WDR5 inhibitor OICR-9429 on T-ALL cells. Remarkably, results revealed that the combination produced a higher cell proliferation arrest and an increased apoptosis compared with the single drug control in three different leukemic T-ALL cell lines. Thus, they aimed to analyze this synergy in vivo and observed that the combination of CX-4945 with OICR-9429 significantly prolonged survival of the mouse models, reduced spleen size and weight, and decreased percentage of human T-ALL cells in both the spleen and the BM compared to single treatment controls. Remarkably, similar results were observed when they performed these experiments using human T-ALL cells from three different patients. Supporting these results, they observed that tumors from mice in the combination group had significant downregulation of WDR5, ATAD2 and cell cycle regulation genes compared with single treatment controls. ATAD2 is a non-canonical ATP-dependent histone chaperone systematically upregulated in various cancers and associated with multiple oncogenic transcription factors 10. Interestingly, when authors overlapped the differentially expressed genes (DEGs) of RNA-seq data in T-ALL cells treated with CX-4945 with that of OICR-9429, they observed that ATAD2 was one of the top downregulated DEGs. These results were confirmed by the fact that the treatment with CX-4945, OICR-9429, or in combination significantly downregulated expression of ATAD2 in three T-ALL cell lines in vitro and in n=4 mice models in vivo. Besides, luciferase reporter assay revealed that overexpressed WDR5 significantly increased the activity of the ATAD2 promoter which was consistent with the enrichment of WDR5. Remarkably, CX-4945 treatment reduced the enrichment of WDR5 at the promoter region of ATAD2, indicating that ATAD2 is the direct downstream target of WDR5 in T-ALL.
Thus, they further evaluated whether ATAD2 drives the oncogenic effects by promoting cell cycle progress in T-ALL and as expected, ATAD2 silencing significantly inhibited T-ALL cell proliferation, induced G1 phase arrest, and downregulated the expression of cell cycle regulation genes in the T-ALL cells compared with control cells. Next, the engraftment of ATAD2-silenced cells vs control cells revealed that ATAD2 KD significantly extended survival and reduced the spleen size and weight, and the percentage of human T-ALL cells in the spleen and BM in vivo. Moreover, higher ATAD2 expression was detected in T-ALL cohorts vs normal BM controls and, in addition, high ATAD2 expression was associated with a significantly higher relapse rate and cell cycle activation in the T-ALL patient cohort.
In conclusion, Han Q. and collaborators identified a new WDR5/ATAD2 oncogenic signaling in T-ALL and the regulatory roles of the CK2/IKAROS axis within the signaling. Besides, their data have demonstrated that the combination of CX-4945 with WDR5 inhibitor has a potent synergistic antileukemic efficacy in T-ALL and provides preclinical evidence for dual targeting WDR5/ATAD2 through CK2/IKAROS as a potential option of T-ALL therapy.
References
- Terwilliger T, Abdul-Hay M. Acute (2017) lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7:e577. PMID: 28665419 DOI: 10.1038/bcj.2017.53. ↩
- Gianni F, Belver L, Ferrando A. (2020) The Genetics and Mechanisms of T-Cell Acute Lymphoblastic Leukemia. Cold Spring Harb Perspect Med. 10:a035246. PMID: 31570389 DOI: 10.1101/cshperspect.a035246. ↩
- Cordo’ V, van der Zwet JCG, Canté-Barrett K, Pieters R, Meijerink JPP. (2020) T-cell Acute Lymphoblastic Leukemia: A Roadmap to Targeted Therapies. Blood Cancer Discov. 2020;2:19-31. PMID: 34661151 DOI: 10.1158/2643-3230.BCD-20-0093. ↩
- Cao F, Townsend EC, Karatas H, Xu J, Li L, Lee S, Liu L, Chen Y et al. (2013) Targeting MLL1 H3K4 methyltransferase activity in mixed-lineage leukemia. Mol Cell. 2014;53:247-61. PMID: 24389101 DOI: 10.1016/j.molcel.2013.12.001. ↩
- Han Q, Gu Y, Xiang H, Zhang L, Wang Y, Yang C, Li J, Steiner C et al. (2025) Targeting WDR5/ATAD2 signaling by the CK2/IKAROS axis demonstrates therapeutic efficacy in T-ALL. Blood. 2025;145:1407-1421. DOI: 10.1182/blood.2024024130. ↩
- Zhang J, Zhou Q, Xie K, Cheng L, Peng S, Xie R, Liu L, Zhang Y et al. (2021) Targeting WD repeat domain 5 enhances chemosensitivity and inhibits proliferation and programmed death-ligand 1 expression in bladder cancer. J Exp Clin Cancer Res. 2021;40:203. PMID: 34154613 DOI: 10.1186/s13046-021-01989-5. ↩
- Götz C, Montenarh M. (2017) Protein kinase CK2 in development and differentiation Biomed Rep. 2017;6:127-133. PMID: 28357063 doi: 10.3892/br.2016.829. ↩
- Song C, Gowda C, Pan X, Ding Y, Tong Y, Tan BH, Wang H, Muthusami S et al. (2015) Targeting casein kinase II restores Ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood. 2015;126:1813-22. PMID: 26219304 DOI: 10.1182/blood-2015-06-651505. ↩
- Song C, Ge Z, Ding Y, Tan BH, Desai D, Gowda K, Amin S, Gowda R et al. (2020) IKAROS and CK2 regulate expression of BCL-XL and chemosensitivity in high-risk B-cell acute lymphoblastic leukemia. Blood. 2020;136:1520-1534. PMID: 32396934 DOI: 10.1182/blood.2019002655. ↩
- Nayak A, Dutta M, Roychowdhury A. (2021) Emerging oncogene ATAD2: Signaling cascades and therapeutic initiatives. Life Sci. 2021;276:119322. PMID: 33711386 DOI: 10.1016/j.lfs.2021.119322. ↩