Towards the generation of tubers in non-tuberizing plants: Can a tomato plant make potatoes?

Some plants have the capacity to develop tubers. Tubers are storage organs that serve as a survival strategy to better cope with adverse environmental conditions such as dry periods and cold. Tubers are sometimes also a means of asexual reproduction. In fact, tubers can persist in the soil during unfavorable conditions and generate a new plant during the favorable season. Tubers vary in their organ of origin; most “famous” tubers are formed from the roots like carrots and potatoes but they can also be formed from other organs like the less known yams or kohlrabi. Tubers accumulate a great quantity of carbohydrates, mainly in the form of starch, which makes them an excellent complement for human dietary needs. In fact, the potato is the third crop in worldwide economic importance after wheat and rice1.
Very little is known about how tuber formation is initiated and why their formation is restricted to a few plant species. For example, tomato plants (Solanum lycopersicum), which are evolutionarily closely related to the potato (Solanum tuberosum), are not able to form tubers. Due to the importance of tubers, for many years scientists and plant breeders have tried hard to learn more about tuber formation and to use breeding techniques to try to transfer the tuber forming capacity of potato plants to non-tuberizing plants such as the tomato.
For example, the german scientist George Melchers fused in 1970s protoplasts of potato and tomato to generate plant hybrids that he named “tomoffel”. Unfortunately these plants did not develop fruits and were unfertile (Abelenda and Prat, 2013). The BBC reported, on the 26th of September, the commercialization of a plant that produces both tomatoes and potatoes named “TomTato” by the Thomson and Morgan company based in Ipswich (United Kingdom) (Figure 1) The director of the company claimed that this was achieved thanks to more than a decade of work by grafting a tomato shoot into a potato detopped plant. One week after the “TomTato” release to the market, the Incredible Edibles company from New Zealand also launched a similar product, the “Potato Tom”.
In June, an article published in Current Biology by Eviatar-Ribak et al2, from the Israel Institute of Technology in Haifa, showed in a very smart way, how the hormones cytokinins (CKs) are involved in the tuberization process. The authors showed that a CK signaling pathway activated by the tomato LONELY GUY1 gene (TLOG1) is sufficient to confer to non-tuberizing tomato plants the ability to form tuber-like organs that the authors named tomato minitubers (TMTs, Figure2). TLOG1 gene encodes the LOG1 enzyme that catalyzes the conversion of CK precursors to biologically active CKs. The authors overexpressed the TLOG1 gene by transforming tomato plants with this gene under the control of a very active promoter (the 35S cauliflower mosaic virus promoter). This approach allowed the generation of tomato plants with increased levels of endogenous CKs that surprisingly generated the formation of TMTs.
TMTs are equivalent to potato aerial tubers that have been described in potato field under flooding (Figure 2). Both the TMTs and the potato aerial-tubers develop two small leaves. TMTs “sprouted” and the emerging leaves gave rise to new TMTs (Figure 2). The overexpression of TLOG1 also had other pleiotropic or “collateral” effects that the authors named as “TLOG1 syndrome”. These effects were, among others, late flowering, attenuated vertical growth and deep green simplified leaves that senesced more slowly than the wild type leaves (Figures 2 and 3). To further decipher the mechanisms underlying TMTs formation, the authors performed a transcriptomic, whole genome gene expression, analysis of TMTs by mRNA sequencing and they observed that gene expression in this tissue was very similar to that of potato aerial tubers.
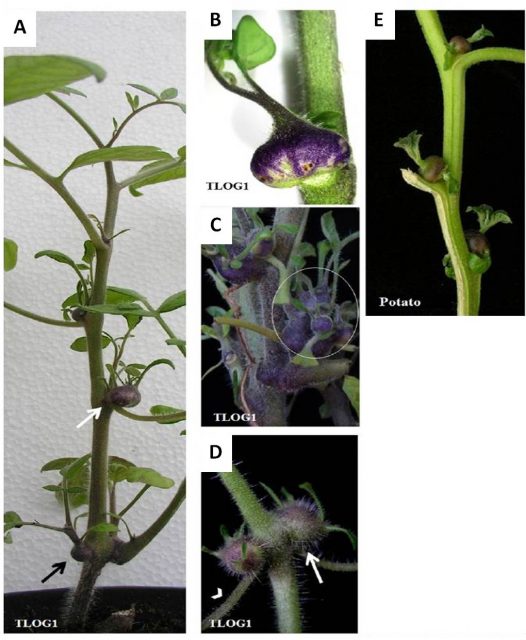
A) Ectopic branching and early TMTs in TLOG1 seedlings.
B) Basal TMTs (arrows) along the primary shoot of a young TLOG1 plant.
C) A TMT with two expanding leaves and a suppressed apex.
D) Chain tubers in TLOG1 plants.
E) TMTs in the CXMs of TLOG1 plants grown in artificial culture medium.
F) An aerial tuber in a stressed potato plant.
Notably, TLOG1 plants only developed the TMTs from the axillary meristems of the basal “juvenile” leaves (Figure 2). This characteristic, together with the fact that TLOG1 plants had late flowering, led the authors to think that the microRNA miR156 could be involved in TLOG1 regulation, as miR156 function had been previously related to flowering and juvenility in Arabidopsis and maize. In this sense, crossing tomato plants overexpressing TLOG1 with plants overexpressing the miR156 generated tomato plants with TMTs not only at the basal leaves but at every nodal bud (Figure 3). Moreover, the miR156 overexpressing plants also produced TMTs under certain environmental conditions, suggesting that CKs and the miR156 could be working together in the regulation of tuberization (Figure 3).
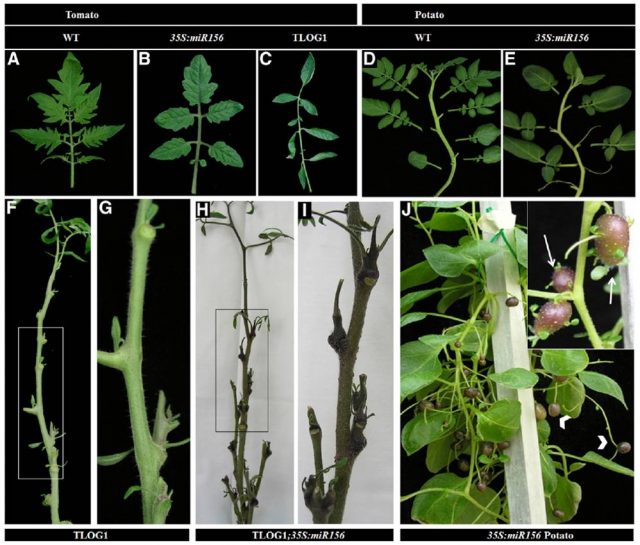
(A) A compound leaf of wild type tomato.
(B) A leaf of a 35S:miR156 tomato plant.
(C) A leaf of a TLOG1 tomato plant.
(D) WT potato shoot. Note the age-dependent changes in leaf morphology along the shoot.
(E) A shoot of a 35S:miR156 potato plant.
(F and G) An upper part of a TLOG1 shoot.
(H and I) miR156 promotes TMTs in upper nodes of TLOG1 plants.
(J) Tuber-bearing aerial stolons (arrows) in a 35S:miR156 transgenic potato plant.
Finally, the overall results of the work from Eviatar-Ribak et al., provide a novel clue for tuber initiation through the discovery of CKs and miR156 involvement in this process and also open the gateway of designing stable new plants wherein the combination of fruits and tubers can be achieved.
References
- Abelenda J.A. & Prat S. (2013). Cytokinins: Determinants of Sink Storage Ability, Current Biology, 23 (13) R561-R563. DOI: 10.1016/j.cub.2013.05.020 ↩
- Eviatar-Ribak T., Shalit-Kaneh A., Chappell-Maor L., Amsellem Z., Eshed Y. & Lifschitz E. (2013). A Cytokinin-Activating Enzyme Promotes Tuber Formation in Tomato, Current Biology, 23 (12) 1057-1064. DOI: 10.1016/j.cub.2013.04.061 ↩