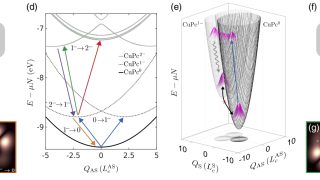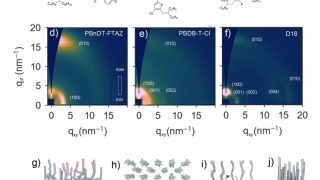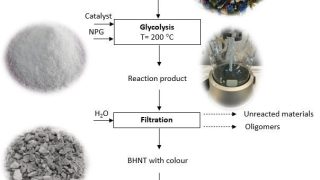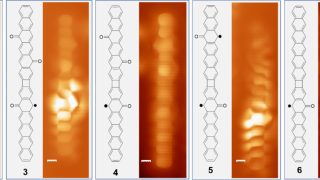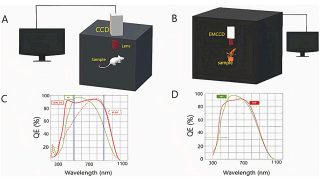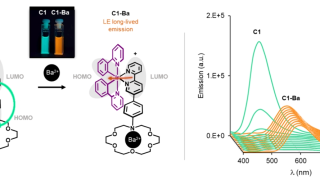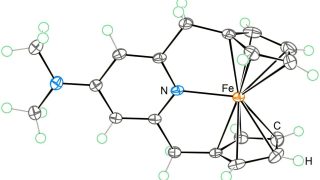
From 18- to 20-electron ferrocene derivatives
For more than a century, a well-known 18-electron rule has guided the field of organometallic chemistry. Now, researchers at Okinawa Institute of Science and Technology (OIST), in collaboration with scientists from Germany, Russia, and Japan, have successfully synthesized a novel organometallic compound that challenges this longstanding principle. They have created a stable 20-electron derivative of […]