Eat healthily, for your children’s sake!
Eat healthily, for your children’s sake!

Nowadays we are more aware than ever about the relevance of eating a balanced and assorted diet. However, in the more industrialized countries obesity has become almost epidemic, and it is a condition that lies at the base of many different health issues, being the cardiovascular complications probably the most obvious. But also immunological and even neurological defects are being assumed, in many cases, as a consequence of a diet rich in fats, usually correlating with a privation of other nutrients. Thus, although we seem to know what we should eat, our modern lives rhythms do not make it easy to keep on the healthy track, and the problems derived from this behavior are only beginning to be understood. That is why I found extremely interesting the topic of today’s article, although in the end, after reading the whole work, I felt a bit disappointed about the lack of answers to the many and interesting questions that it seems to open. But science is more about questions than about answers, so let’s discuss a little bit and see what interesting facts we can learn from it.
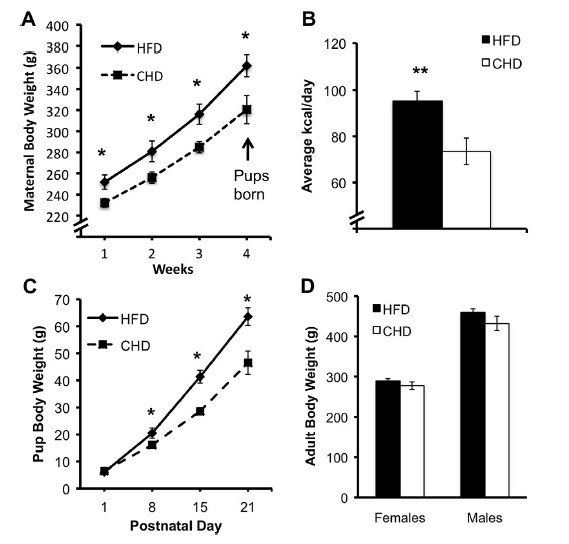
Sasaki and collaborators have studied the effect of drastically changing the type of diet on laboratory rats. The results, published in Neuroscience 1, consist on a description of several treats related to animal behavior as well as a series of analysis at the molecular level. The interesting fact and the key question is that the subjects of study were not the differentially fed rats, but… their descendants. It is not a novel observation that, in mammals, environmental factors affect the development of the offspring, before and after birth. The particular area where the authors focused their attention was the hypothalamic-pituitary-adrenal axis (HPA axis, from now on). These three anatomic regions are involved in the production and regulation of substances that mediate endocrine response to stress. To make it clearer: the circuit involving certain brain areas that recognize stress situations and respond to them by generating a series of neurotransmitters and hormones, which in the end prepare the body for a concrete response towards the stressing stimulus. Since maternal stress had previously been related to alterations in the offspring’s behavior, the authors wondered whether a condition of obesity could have a similar effect. To answer this question, the experimental design consisted in two groups of rats, which were fed with either a type of chow rich in carbohydrates or in fats (about 60% of the total components, in both cases). The differential feeding took place from 4 weeks prior mating and throughout pregnancy and lactation. Once the little pups were grown into adult rats, they were fed on the normal chow diet. The data were collected attending to two different aspects: a behavioral set of tests, and a biochemical and molecular characterization of blood and brain parameters. It is interesting to note that, although the pups from the fat-eating mothers were heavier than the ones from normal chow-eating, once they got to adulthood the body weights from both groups were balanced. This led researchers to assume that any changes observed in the parameters of study were not due to a condition of obesity of the experimental subjects, but to the “legacy” of their mothers.

The rats born from the fat-eating mothers displayed an altered response in the behavioral tests, which was assumed to be a consequence of high levels of anxiety. Among other complicated tests, a curious experiment was to count the number of excrements produced by the rats when altering their light-dark cycles. A very obvious, but efficient, measure of anxiety: the rats from fat-eating mothers were especially anxious. So they produced more shit. Science is beautiful, isn’t it?
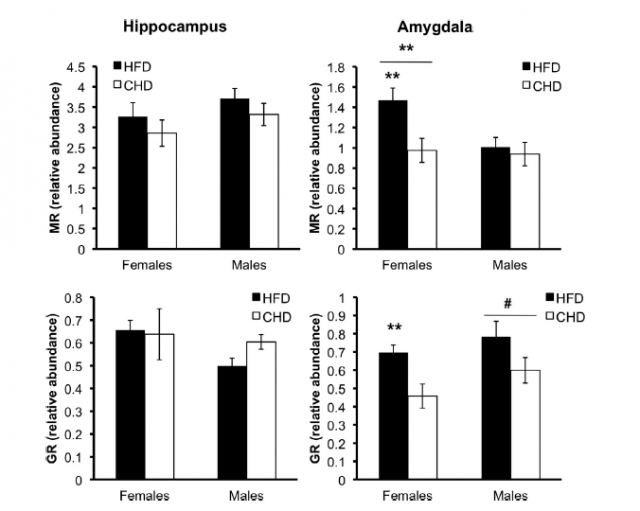
To correlate the observed altered anxiety levels with the differential feeding of the mother rats, the authors focused their attention into corticosteroids, molecules that mediate stress response (among many other processes) and which trigger other molecular pathways that usually affect the inflammatory response. Although some corticosteroids levels were affected, they were not in all the brain areas examined, and sometimes the effect was sex-dependent. This partial effect was also obtained for the rest of markers used to analyze the effects of differential diets: some genes were overexpressed in the rats from fat-eating mothers, but some other genes were not. For instance, they also registered and increase in the expression levels of pro-inflammatory response genes, as well as a differential expression of anti-inflammatory ones. This unbalance in inflammatory response is proposed to be a consequence of the altered corticosteroid levels, and taking together all the data, it seems reasonable that the alterations studied in these experimental rats are all directly related to the aforementioned HPA axis: corticosteroid levels are not well regulated and trigger an altered balance of the inflammatory activity, something which already had been found in other works that correlate obesity with altered inflammatory response. The interesting fact here, again, is that the stress-causing condition is not an actual environmental factor of the studied individuals, but of their mothers before and shortly after giving birth to them.
However, this correlation is far from being totally explained as a causative process. The differences in dietary composition for both groups of rats may result in several other reactions that where not examined by the authors, and as they honestly state in the discussion section of the paper, the differences between both diets go beyond the simple dichotomy fat/ no fat, since the “high-fat” chow is also containing almost double amount of refined sugars than the “normal” chow. This is not a trivial issue and the determination of effects due to high concentration of fats or otherwise the contribution of refined sugars is still missing. In any case, the fact that changes in the physiology of a mother influenced by environmental factors can affect the offspring’s biology in adulthood, may seem something amazing. Even subtle effects as the ones depicted in this work. Probably the reader familiar to modern advances in biology must be meditating a word: epigenetics. It is very possible that the way the molecular changes produced in the mothers during their exposition to a high-fat diet were not only at a metabolic, but also genetic level, making modifications in the way some genes are regulated. Besides, these genetic alterations are transmitted to the offspring. That is basically what epigenetic regulation is about: heritable treats not due to changes in the genetic sequence but in their regulation. Is this the case of the rats fed with a high-fat diet and their anxious puppies? Only a detailed readout on the epigenetic modifications of those genes that showed a clear modification in their expression levels would hold the key to the question. There are already other works where a correlation between high fat content in diet and molecular alterations of the machinery that regulates epigenetic imprinting of certain genes has been reported 2, and an increasing amount of evidence pointing in this direction is accumulating. Since this is an emerging field that is developing extremely quickly, the answer may not be far away, and it would be very useful to find out if these effects observed in experimental animals are extrapolable to us, humans which fancy devouring greasy foods. In the meanwhile, we better take care of what we eat, not only because of the creepy consequences suffered and exposed by the creator of Super size me, but taking into account the possible genetic legacy we may be inflicting to our descendants.
References
- Sasaki A., de Vega W.C., St-Cyr S., Pan P. & McGowan P.O. (2013). Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood, Neuroscience, 240 1-12. DOI: 10.1016/j.neuroscience.2013.02.044 ↩
- Langie S.A.S., Achterfeldt S., Gorniak J.P., Halley-Hogg K.J.A., Oxley D., van Schooten F.J., Godschalk R.W.L., McKay J.A. & Mathers J.C. (2013). Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring, The FASEB Journal, 27 (8) 3323-3334. DOI: 10.1096/fj.12-224121 ↩
5 comments
[…] esos efectos son no sólo endocrinos sino también cognitivos. Los detalles los encontramos en Eat healthy, for your children’s sake! de Carlos […]
[…] Eat healthily, for your children's sake! – Mapping Ignorance Thus, although we seem to know what we should eat, our modern lives rhythms do not make it easy to keep on the healthy track, and the problems derived from […]
These results are somehow expected, but more than welcome because it actually confirms how important is diet, not just for your own health but also for the next generations. It also may raise the possibility that “available” food may have been an important factor in driving evolution of organisms because different nutrients may have been available in different past periods of time. Good one mate!!
I’m glad you liked the post, and surely if the conclusions are right, this would have very interesting implicatons from an evolutionary perspective, too. However, it should be noted that the mechanisms involved are not clear at all, and in order to be sure at what extent epigenetic mechanisms are taking place here, much mlore work should be performed in this direction. Thanks for the comment!
[…] Eat healthily, for your children’s sake! […]