Brain tumours and the lack of molecular editing
When we talk about nucleic acids we generally think of DNA and RNA that code for proteins, and when we talk about brain cells we tend to think of neurons and their nerve impulse transmission. However, it is not news that the functions of both non-coding genetic material and non-transmitting cells in the brain are vital for the normal functioning of living organisms. Lately, a paper has been published on the relationship between them with some promising observations.
microRNAs (miRNAs) are short pieces of RNA (around 22 nucleotide long) that do not code for proteins, but are still important for regulating gene expression since they are responsible for adjusting, or fine-tuning, up to 30 percent of all protein-encoding genes in mammals. This fine-tuning can happen in various ways that involve changes (“editing”) to the RNA, like insertion, deletion, or chemical transformation of nucleotides. The objective of these processes is to change the information encoded by the genome, playing a crucial role in the regulation of many cellular pathways including proliferation, differentiation and development, by silencing RNA targets by either inhibiting their translation or promoting their degradation.1 Since miRNAs can act as oncogenes or tumor suppressors, fluctuations of their expression are important factors in both normal and pathological conditions.
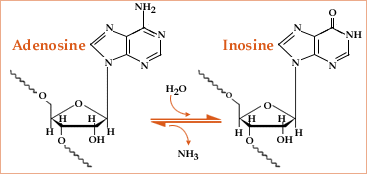
The main form of RNA editing in mammals is “A-to-I editing”, which is vital for the normal function of living organisms2 and is enriched in the brain.3 It consists of the deamination of adenosine (A) nucleotides in an RNA chain to give inosine (I) nucleotides after hydrolysis, and is catalysed by enzymes known as ADARs (adenosine deaminases acting on RNA). A study from 2012 on different development stages has also shown that A-to-I editing in miRNA increases during mouse brain development.4 Problems with microRNA editing, which lead to changes in gene expression, have already been linked to several types of cancer; for example, it has been observed that the activity of ADAR2 is slowly lost during the progression of astrocytoma (a type of brain cancer affecting astrocytes) in children.5
A recent study that found tissue samples from brain cancer patients show a lack of miRNA editing is particularly exciting and hopefully promising: recently, Deepanjan Paul and his colleagues at the Institute of Genomics & Integrative Biology in Delhi have published a paper in which they show how they found a link between brain tumours and the lack of A-to-I editing6 when examining samples of brains affected by glioblastoma multiforme (GBM), one of the most aggresive types of brain cancer that targets glial cells. Like in most brain cancers, complete removal of the tumour is not always possible without damaging important neighbouring areas due to its location, and despite the maximum use of radiotherapy or chemotherapy, the tumour cells are very resistant and the tumour usually recurs with a poor prognosis.
The team examined the normal miRNA editing spectrum in 13 different types of healthy human tissue such as brain, lung, skin, kidney, ovary, heart and liver, and found the healthy brain to have the highest amount of miRNA editing. In subsequent experiments, after observing samples of brain tissue affected by GBM and comparing them to samples of the healthy brain, Paul and his colleagues observed that A-to-I editing happens much more often in the miRNAs of healthy brains than of brains affected by this type of cancer.
This research is at too early a stage to jump to conclusions, but this discovery is exciting as it suggests a relationship between faulty miRNA editing and abnormal uncontrolled cell growth. The importance of this finding lies in the fact that it has established a new starting point for research into these types of tumours, which could eventually help us learn more about how they originate at the biochemical level. In the future, this could eventually help researchers find strategies to slow down the progression of brain tumours, or even to prevent them from growing in the first place, possibly by using techniques like CRISPR.
References:
1 Bartel DP. “MicroRNAs: genomics, biogenesis, mechanism, and function”. Cell 116, 281 (2004). http://dx.doi.org/10.1016/S0092-8674(04)00045-5
2 Keegan, L. P., et al. “The many roles of an RNA editor”. Nature reviews. Genetics 2, 869 (2001). doi:10.1038/35098584
3 Paul, M. S. & Bass, B. L. “Inosine exists in mRNA at tissue-specific levels and is most abundant in brain mRNA”. The EMBO journal 17, 1120 (1998). doi:10.1093/emboj/17.4.1120
4 Ekdahl, Y. et al. “A-to-I editing of microRNAs in the mammalian brain increases during development”. Genome research 22, 1477 (2012). doi:10.1101/gr.131912.111
5 Cenci C, et al. “Down-regulation of RNA editing in pediatric astrocytomas: ADAR2 editing activity inhibits cell migration and proliferation”. Journal of Biological Chemistry 283, 7251 (2008). doi: 10.1074/jbc.M708316200
6 Paul, D. et al. “A-to-I editing in human miRNAs is enriched in seed sequence, influenced by sequence contexts and significantly hypoedited in glioblastoma multiforme”. Nature Scientific Reports 7, 2466 (2017). doi:10.1038/s41598-017-02397-6
2 comments
[…] MikroRNAren edizio arazoak zenbait tumore motarekin daude lotuta. Ikerketa berri batek etorkizun handiko emaitzak lortu ditu garun tumoreekiko. Isabel Pérez Castrok azaltzen du Brain tumours and the lack of molecular editing. […]
[…] Los problemas en la edición del microARN están asociados ha distints tipos de tumores. Una reciente investigación arroja resultados muy prometedores para los tumores cerebrales. Isabel Pérez Castro informa en Brain tumours and the lack of molecular editing. […]