Early research on the origin of life. The Miller experiment.
“It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present.— But if (& oh what a big if) we could conceive in some warm little pond with all sorts of ammonia & phosphoric salts, light, heat, electricity etc present, that a protein compound was chemically formed, ready to undergo still more complex changes, at the present day such matter would be instantly devoured, or absorbed, which would not have been the case before living creatures were formed.”
Charles Darwin, in a letter to Joseph D. Hooker (February 1871)
The chemical processes that led to the origin of life have always fascinated scientists. A few weeks ago we talked about the most recent advances in the research on this topic (https://mappingignorance.org/2018/07/04/a-new-theory-on-the-early-building-blocks-of-life/). Now, we will focus on how research on the origin of life was carried away in its beginnings, and on an experiment that took more than fifty years to be completed.
It was already eighty years ago, in 1938,1 that it was first suggested that the early atmosphere of Earth could have been formed by methane, ammonia, water, and hydrogen, and that the organic molecules of life were first formed from them rather than from the carbon dioxide, nitrogen, oxygen and water that can be found in the atmosphere we know now. In order to test this hypothesis, Stanley L. Miller published did some experiments and published a papers in 19532 describing the synthesis of amino acids caused by a spark in a mixture of gases supposed to be present in the early Earth atmosphere.
Miller, then an undergraduate, and his supervisor Harold Urey designed a device to mimic the conditions on early Earth: it held a mix of methane, ammonia and hydrogen gases, a water pool to represent the ocean, and electrodes that delivered an electric current into the gas mixture, simulating lightning. After allowing the experiment to run for one week, Miller observed that the water had become coloured and turbid, and when its contents were analysed, it was found that organic compounds had been formed. Among them, five aminoacids were identified.
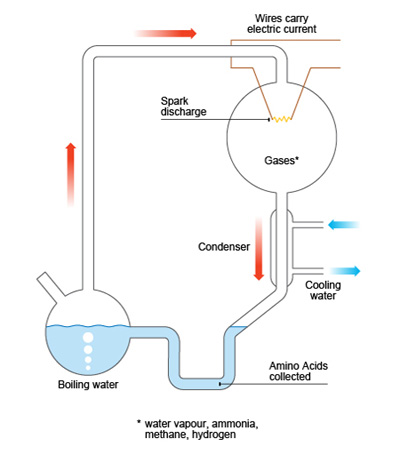
Since the experiments were carried away in sealed systems and the water was boiling, Miller was sure that none of the molecules could have not been made by microorganisms. Also, the work-up with HgCl2, Ba(OH)2, H2SO4 made the presence of any living organisms during the analysis impossible. This confirmed that the building blocks of life could be synthesized from very simple, small molecules, and suggested that the “warm little pond” Darwin had talked more than eighty years before was a very likely scenario in which storms with lightning may have played an important role. Later, in 1955, Miller reported the identification of other compounds in the water mixture, such as carboxylic and hydroxy acids.3 But he would not have been able to identify anything present at very low levels due to the limitations of the techniques he was using.
Miller carried on with his research on this topic during the 1950s but, for some reason, no more results were published. However, following good research practice, he kept all his samples stored in vials, clearly labelled and with references to his lab notes, and it was not difficult for one of his former students, Jeffrey L. Bada, to carry on with the work on the samples he found after Miller’s death in 2007.
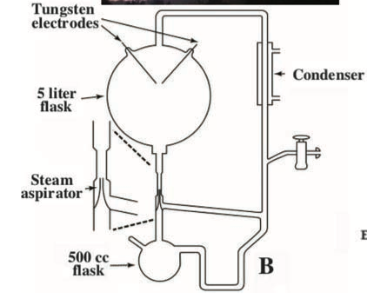
It turned out that the next steps in Miller’s research consisted in adding H2S to the mixture of gases,4 and/or in using a “volcanic apparatus”: a more complex version of his original device, which differend from it in that it included an aspirator that injected steam into the electric discharge chamber, simulating a volcanic eruption.
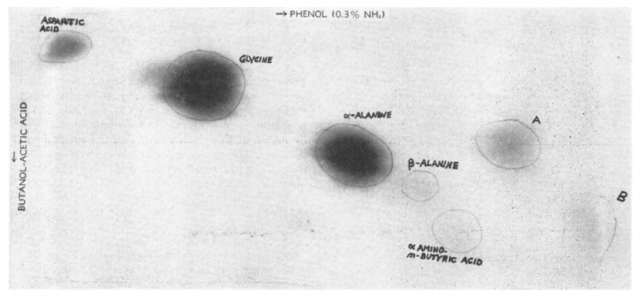
Since the techniques available now are much more sensitive, Bada and his students decided to analyse the contents of the vials and found that the mixtures had twenty three aminoacids, far more than the five originally described, including a few sulphur-containing ones originated in the experiments performed with H2S. They also found other sulfur-containing organic compounds, and products of the degradation of aminoacids -not surprising, considering that the experiment took 53 years to be completed.
The authors suggest that, despite the gas mixture with H2S Miller tested in 1958 may not have been ubiquitous throughout the primitive atmosphere, it might have been prominent in certain areas (for example, near volcanoes), where these gases may have played a vital role in the localized synthesis of some of the first terrestrial organic compounds. This suggests that a mixture of oxidized and reduced gases, including H2S, may have aided the synthesis of amino acids and amines on the primitive Earth.
It is important to note that, on his original paper from 1953, Miller made clear that his experiment attempted to recreate the primitive atmosphere of the earth rather than to obtain the optimum conditions for the formation of aminoacids, although he suggested that a similar device and a different composition of the gas mixture could be useful for the industrial production of aminoacids. This is yet another example of how research on basic science with apparently no practical applications, besides being fascinating in itself, has the potential of being behind industrial, money-making applications.
References:
1 Oparin, A. I. The Origin of Life. New York: Macmillan (1938).
2 Miller, S. L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 117, 528 (1953).
3 Miller, S. L. Production of some organic compounds under possible primitive Earth conditions. JACS 77, 2351 (1955).
4Parker ET, et al. Prebiotic synthesis of methionine and other sulfur-containing organic compounds on the primitive Earth: A contemporary reassessment of an unpublished 1958 Stanley Miller experiment. Origins Life Evol. Biosph., 201 (2011).
DOI: 10.1007/ s11084-010-9228-8.
5 Johnson, A. P. et al. The Miller Volcanic Spark Discharge Experiment. Science 322, 404 (2008).
DOI: 10.1126/science.1161527
1 comment
[…] Mamitsuak izan ziren bizitzaren jatorri abiotikoaren inguruko lehen esperimentuak. Isabel Pérez Castrok azaltzen du Early research on the origin of life. The Miller experiment. […]