The thermodynamic limits of lightness
Introducing a gaseous phase into a solid matrix is one of the most common strategies to reduce weight and increase thermal insulation in a wide range of materials, from ceramics to metals and polymers. How to produce these two-phase materials is mostly determined by the nature of the solid matrix and has led to the development of a vast field of knowledge in materials science: the field of cellular materials, also commonly referred to as foams.
Cellular materials have two key advantages in terms of both material and energy sustainability: on the one hand, replacing part of the solid material with gas definitely reduces the final weight and decreases the use of raw materials; on the other hand, introducing a gaseous phase decreases the thermal conductivity of the final product, leading to a reduction in the energy consumption in applications such as building insulation. This is not a minor issue in Europe, where 75% of the building stock is energy inefficient1 and up to 64% of the household energy consumption is dedicated to heating2. The development of high-performance thermal insulation materials will be certainly relevant to achieve a remarkable cut in greenhouse gas emissions in the next few years.
In this article we are going to focus on this characteristic feature of cellular materials as good thermal insulators, which lies on the fact that the thermal conductivity of the gas is considerably lower than that of the solid material. This way, the less solid matter you have in a cellular material, the better insulation you get, which is equivalent to say that what you should look for is the lowest possible density. Some very popular polymeric foams used in building insulation such as expanded polystyrene (EPS) and polyurethane (PU) are based on this principle, and usually have densities in the order of 20-40 kg/m3 and thermal conductivities of 0.03-0.04 W m-1 K-1.
As it is often the case, nature is no stranger to this mechanism and presents countless examples of insulation materials based on this principle of ‘trapping’ air among a solid phase. That is the case, for instance, of some animals living in extremely cold environments (see the penguin feathers in the image below), where the use of fur or feathers represents this basic strategy of trapping layers of warm air near the skin. Or the many plant-base materials which are being rediscovered for building insulation, such as cork, wood wool, straw bales, cotton, flax or hemp3.

This basic principle has led to the refinement of the techniques trying to achieve stable cellular structures with high-performance thermal insulation properties. One of the most popular material in this group is aerogel, a two-phase ultralight material with a thermal conductivity below that of air itself (0.015 Wm-1K-1 vs 0.024 Wm-1K-1). Aerogels, also known as ‘solid air’, consist of an intricate network of solid filaments filled with pores with sizes in the range of the nanoscale. In these structures, the gas phase can reach up to 99.8% of the total volume, making the solid contribution to the thermal conductivity almost negligible. But, how can aerogels have thermal conductivities below that of the gas they contain? Well, this is caused by a well-known phenomenon called the Knudsen effect, which is the ‘most wanted’ guy in the thermal insulation research field.
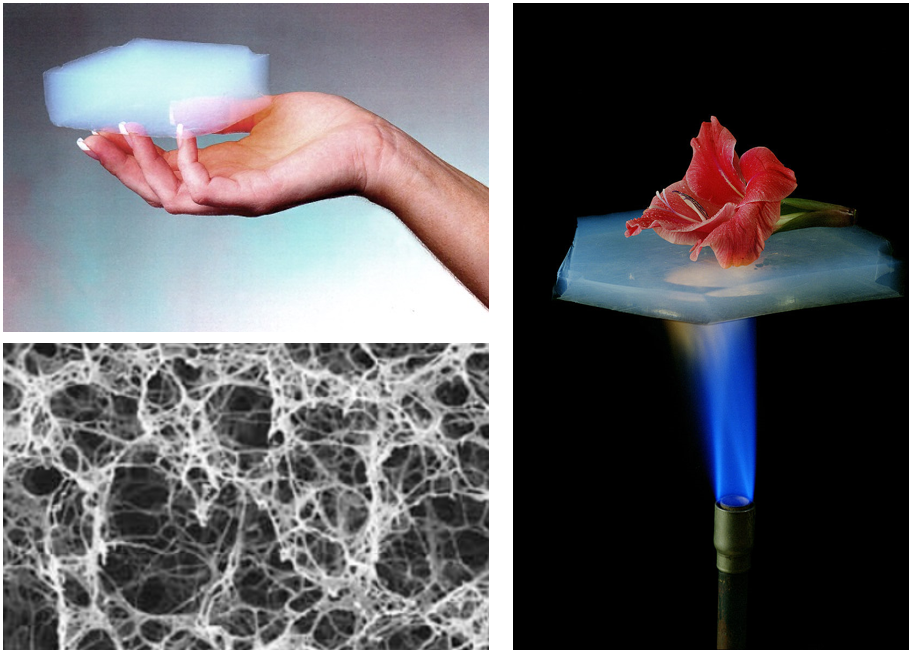
The Knudsen effect is based on a parameter called mean free path, which is the average distance travelled by a moving gas molecule between successive collisions with other gas molecules. In air, this parameter is close to 70nm at normal conditions. In an ultralight cellular material, heat diffusion is mainly caused by the constant exchanges of energy caused by the gas molecules collisions within the cells but, if the size of the cell is smaller than the mean free path of the gas molecules, interactions between these gas molecules are highly restricted and the predominant type of collision will be that of the gas molecules with the cell walls. In this new transport regime, heat cannot be diffused by the gaseous phase and thermal conductivities below that of air are achievable. The Knudsen number (Kn) defines this ratio between the mean free path and the cell size and, in order to achieve highly reduced thermal conductivities, Kn must be greater than one, as represented in the picture below.

What this phenomenon really means in terms of the cellular structure of high-performance thermal insulation materials is that the cell diameter must be reduced to the range of the nanoscale (approx. 10-70nm) while maintaining a very low total density (in the range of 0.1-0.2 g/cm3). These materials are commonly referred to as nanofoams, and their manufacturing at a commercial scale is far from being easy. Considerable advances have been made in polymeric foams using the ‘solid state foaming’ process5, where a gas, usually CO2, saturates the polymer under high pressure conditions and temperatures above the polymer’s glass transition. Once the molecular diffusion of the gas within the polymeric material is ensured, temperature and pressure are rapidly dropped causing cells formation, growth and stabilization.
Solid state foaming has obtained good results at laboratory scale with a variety of polymers, achieving cell sizes in the range of 50-100nm and porosities close to 80% in thin films. But going beyond these limits seems somehow unachievable and the reasons, although thoroughly researched, have not yet been fully explained. However, there is indeed a consensus that the answer to this limitation lies in the cell nucleation phase, that is, in the very beginning of the ‘bubble’ formation. At the moment, macroscopic approaches focusing on the optimization and control of parameters such as the polymer’s rheology have been helpful but limited, and the only thing we know for certain is that creating nanocellular structures in polymers similar to those of aerogels are rarely viable.
The truth is we know little about this crucial moment in which a nanobubble is created and how to freeze that moment and prevent the bubble from either collapsing or growing beyond that size. A recently published study6 could be a big step forward in order to understand this specific issue, and its first hypothesis is that if we want to achieve cell sizes close to molecular sizes, we have to dig deeper into the nature of the nucleation phase, abandoning conventional approaches usually applied to foams as continuous media and considering the discrete nature of the polymeric material.
Estravis and colleagues from the Department of Materials Science and Metallurgy at the University of Cambridge have explored these limits using molecular dynamics, a computer simulation method that analyses the physical movements and interactions of atoms and molecules. They have tried to reproduce how a polymer reacts when it is forced to accommodate a nanometric void, the nanobubble, diving into the thermodynamics of this phenomenon.
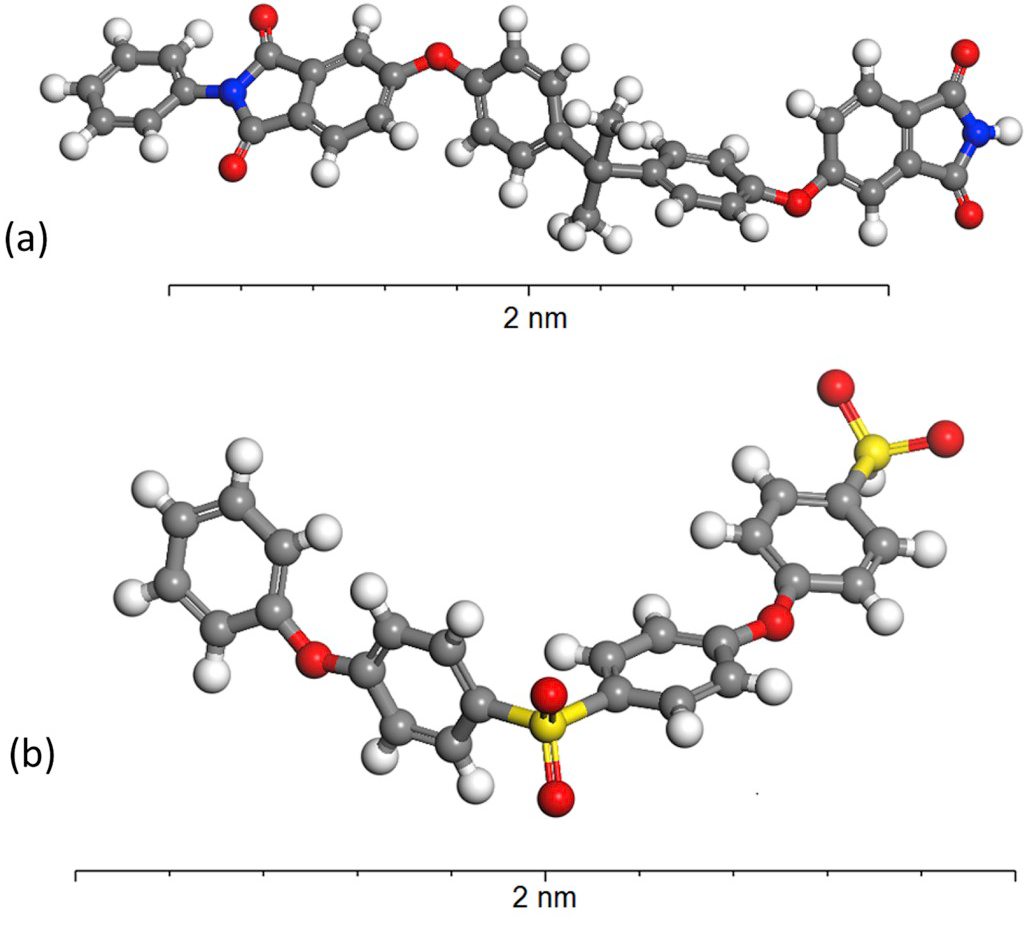
The most innovative approach in this study is that it focuses on the internal pressure of the polymer (defined as the variation in the internal energy derived from volume variations) to analyse why some polymers behave better than others in the stabilization of nanobubbles. The results find that the stability of nanovoids in the range from 0 to 4 nm highly depends on the internal pressure of the specific polymer under study. High values of this parameter prevent the nanovoid from stabilizing within the polymer structure. The results would suggest that if the internal pressure of the polymer exceeds certain value in the first moments of the foaming process, it would prevail the tendency of the polymer to collapse in order to recover the minimum energy configuration, and the nanocellular structure would not be viable. The study successfully represents the internal pressure of several polymers and finds that even polymers with similar molecular structures, such as polyether sulfone (PES) and polyetherimide (PEI), show different behaviour in the stabilization of nanocells, most probably because of the different distance and angle distributions of atoms across the polymeric chains.
Without any doubt, these results get us closer to understand the limiting factors to create cost-effective nanofoams and will enable further improvements in the selection and development of ultralight, high-performance insulating materials. Once again, simulation techniques demonstrate their power to understand materials behaviour unexplained with conventional, experimental techniques. There is still a lot to be done in this field of research, but it seems that we are beginning to understand the thermodynamic limits of lightness.
References:
1 https://ec.europa.eu/info/news/focus-energy-efficiency-buildings-2020-feb-17_en
2 https://ec.europa.eu/eurostat/statistics-explained/index.php/Energy_consumption_in_households
3 Bozsaky D., Nature-Based Thermal Insulation Materials from Renewable Resources – A State-Of-The-Art Review, Slovak Journal of Civil Engineering Vol. 27, 2019, No. 1, 52 – 59; doi: 10.2478/sjce-2019-0008.
4 Zuo L. et al, Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications, Materials 2015, 8, 6806–6848; doi:10.3390/ma8105343.
5 Forest C. et al, Polymer nano-foams for insulating applications prepared from CO2 foaming, Progress in Polymer Science, Vol. 41, 2015, 122-145; doi.org/10.1016/j.progpolymsci.2014.07.001.
6Estravis S. et al, Thermodynamic limits on cell size in the production of stable polymeric nanocellular materials, Polymer, 186 (2020) 122036; doi.org/10.1016/j.polymer.2019.122036.