Electronic bonding network and critical temperature in hydrogen-based superconductors
The capacity of creating an electronic bonding network between localized units would be key to enhance the critical temperature in hydrogen-based superconductors, according to recent results. 1
The field of hydrogen-based superconductivity has progressed enormously since 1968, when Ashcroft first proposed that pressurized hydrogen may become a high-temperature superconductor due to the high energy of its phonons. While the first discovered hydrogen-based superconductors in 1972 presented not very promising critical temperatures (Tc), the more recent experimental discoveries at high pressures show that superconductivity on hydrogen-based compounds can span from few Kelvin to ambient temperature. Ambient temperature should not be understood as that of the North Pole in winter, but closer to that of Vitoria-Gasteiz in autumn: at 267 GPa, a compound formed by sulphur, carbon, and hydrogen showed a Tc of 288 K. Hydrogen-based compounds are thus the best currently available candidates to reach ambient temperature and pressure superconductivity.
The hundreds of compounds predicted to be superconductors by ab initio crystal structure prediction techniques constitute a rich working data set. In order to extract useful information from these data, two main routes are being explored: on the one hand, machine learning methods are starting to be employed to further increase the list of predicted systems, although the obtained new compounds so far do not beat the already known; on the other hand, additional efforts are being invested into classifying these superconductors using simple footprints based on structural, chemical, and electronic properties.
But, even if the footprints for a good superconductor are somewhat clear, we cannot yet rely on simple variables to estimate the superconducting temperatures, clarify the reason for such a broad spectrum of Tc values, and, ultimately, chemically engineer better superconductors. In other words, even though theoretical considerations have been able to guide recent experimental discoveries, the point still is that there is no simple physical-chemical understanding of the properties enhancing the critical temperatures in hydrogen-based systems, hindering the discovery of new compounds with high Tc at low pressures. Now, a team of researchers provides such an understanding.
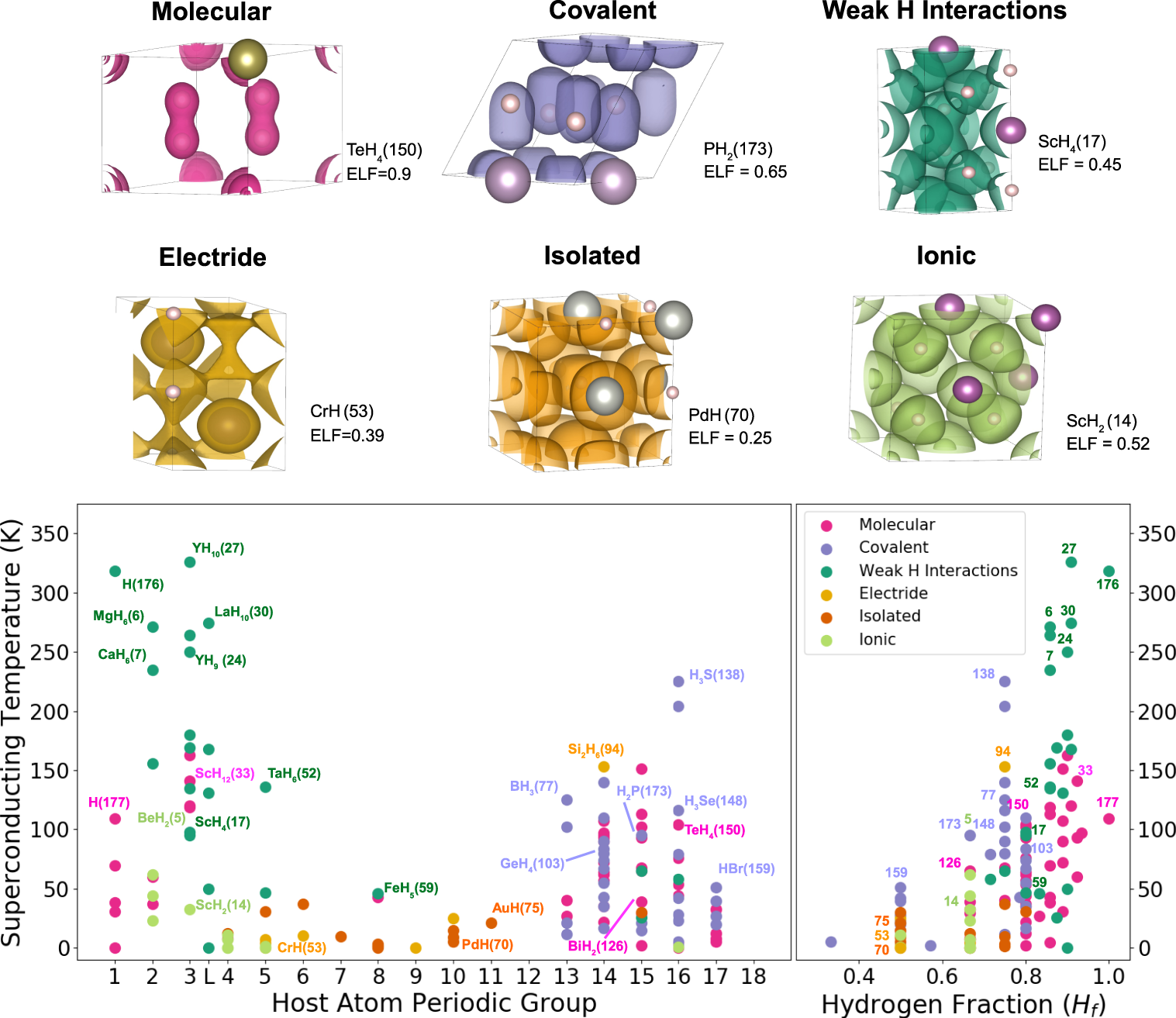
To achieve that, they have investigated the chemical, structural, and electronic properties through ab initio methods based on density functional theory for a set of 178 hydrogen-based superconductors previously predicted in the literature. They focused mainly on chemical bonding descriptors and reviewed the impact on the predicted Tc of many of them: none of them reveals conclusive.
To overcome this, the authors propose a universal descriptor based on the identification of electronic delocalization networks by means of the electron localization function. They define a simple magnitude, the networking value, which is easily obtained. Such quantity reveals useful to have a first estimate of the superconducting critical temperature without performing electron-phonon coupling calculations. It is the first time that such a descriptor is proposed.
The networking value correlates with the predicted critical temperature better than any other descriptor. Classifying the studied compounds according to their bonding nature, such correlation is bonding-type independent, showing a broad scope and generality. Actually, combining the networking value with the hydrogen fraction in the system and the hydrogen contribution to the density of states at the Fermi level, Tc of hydrogen-based compounds can be predicted with an accuracy of about 60 K.
The networking value could reveal strongly insightful in understanding the physics and chemistry of high-temperature superconductivity and, at the same time, it provides paths for chemically engineering better hydrogen-based superconductors, guiding the quest for high- Tc compounds among the vast possibilities offered by ternary compounds.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers.
References
- Belli, F., Novoa, T., Contreras-García, J. & Errea, I. (2021) Strong correlation between electronic bonding network and critical temperature in hydrogen-based superconductors. Nat. Commun. doi: 10.1038/s41467-021-25687-0 ↩