Lysosomes: the Achilles heel of brain macrophages during a stroke
Lysosomes: the Achilles heel of brain macrophages during a stroke
Authors: Ainhoa Plaza-Zabala, Glial Cell Biology Lab, Achucarro Basque Center for Neuroscience, Dpt. of Pharmacology, University of the Basque Country UPV/EHU and Amanda Sierra Glial Cell Biology Lab, Achucarro Basque Center for Neuroscience, Dpt. of Biochemistry and Molecular Biology, University of the Basque Country UPV/EHU, Ikerbasque Foundation
The brain tissue is filled by cells, the living units that enable function; the extracellular matrix, the scaffold wherein cells organize; and the blood vessels, which transport metabolic fuels such as oxygen and nutrients to the brain. Among the brain cells, we find the neurons, highly specialized cells that transmit information; and the glial cells, including astrocytes, oligodendrocytes, and microglia, which perform essential functions to facilitate and optimize information transmission by neurons.
Microglia are the cells that defend and protect the brain from injury. They constitute around 10% of brain cells, and exhibit a small and static cell body surrounded by continuously moving, fine and long processes. These processes are equipped with an array of molecules that respond to chemical and/or physical changes in their environment. After activation of these molecules, microglia engage in diverse actions that eventually support the recovery of tissue balance. For example, microglia clear toxic extracellular detritus in a process known as phagocytosis. This microglial function is essential to maintain brain health. It is then of outmost importance to promote the health and function of microglia in the injured brain.
During ischemic stroke, a vascular plug forms in an artery of the brain, interrupting the blood flow to the brain areas irrigated by the artery. Depending on the time length and intensity of the blood irrigation blockade, the neurons and glial cells of the artery-surrounding tissue suffer from oxygen and nutrient deprivation at different degrees, eventually leading to their death. Due to the high specialization of neurons on transmission of information, neurons are usually the first to die in significant numbers. However, glial cells, and more specifically microglia, also suffer from the deprivation of oxygen and nutrients induced by ischemic stroke.
In a recent original research article 1, we have shown that microglia lose their ability to phagocytose dead neurons in mice and monkey models of ischemic stroke. Phagocytosis of dead neurons is essential to maintain a healthy brain environment. Normally, dead neurons are efficiently recognized, engulfed, and digested by microglia, and leave no remnants behind. The degradation of the phagocytosed cargo is achieved by the delivery of the dead neuron’s pieces to lysosomes, the digestive compartments or organelles of all mammalian cells, including microglia. However, dead neurons that are not efficiently cleared up by microglia lose their integrity and release toxic substances, affecting the health of the surrounding cells 2. This phagocytosis impairment occurs because ischemic stroke reduces the microglial availability of lysosomes.
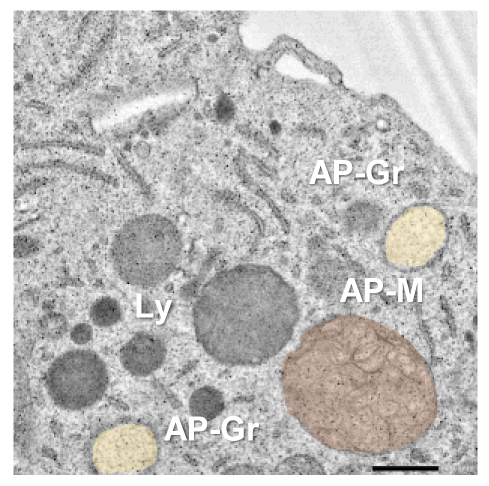
Lysosomes are small to medium-sized, round, and membrane-sealed organelles equipped with all the necessary machinery to degrade any kind of biological macromolecule including carbohydrates, lipids, proteins, and nucleic acids. Therefore, lysosomes not only degrade macromolecules coming from outside the cell (for example, dead neurons removed by microglia through phagocytosis) but also intracellular damaged or no longer needed content. This latter process is known as autophagy (eating one-self), and is stimulated to promote survival under metabolic stress conditions such as oxygen and nutrient deprivation. Interestingly, we have observed that ischemic stroke and the associated lack of oxygen and nutrients, intensifies autophagy in microglia, probably to get rid of damaged proteins and organelles, adapt to the new metabolic scenario, and promote microglial survival. However, autophagy promotion during ischemic stroke does not come without cost for microglia. Indeed, the over-engagement of the autophagic degradation pathway in microglia leads to a significant depletion of lysosomes, which are no longer available for the clearance of phagocytic material such as dead neurons.
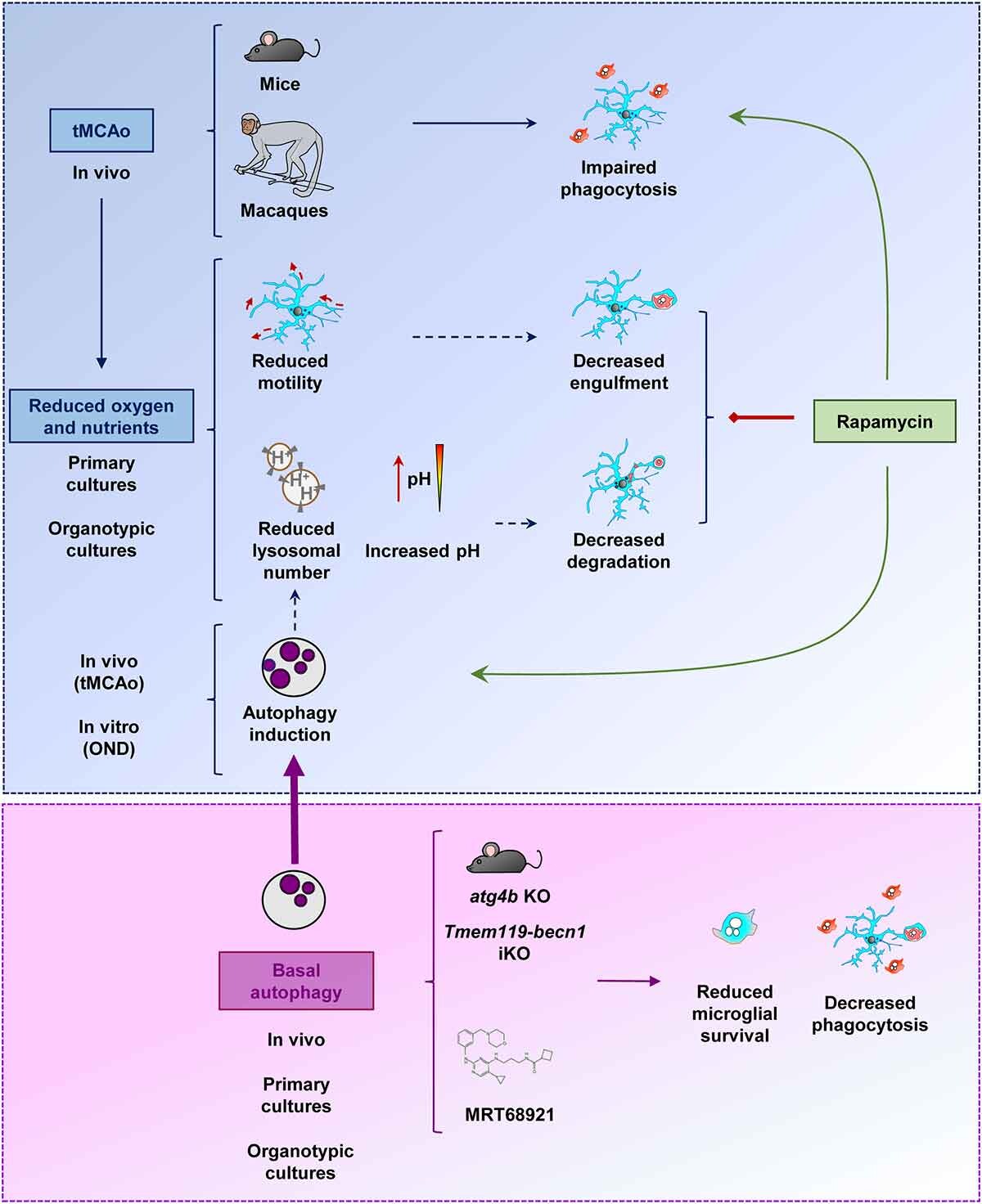
Microglial phagocytosis blockade on ischemic stroke has consequences on the amount of toxic material that accumulates in the brain, and worsens the evolution of the disease. Then, it could be useful to recover the phagocytic activity of microglia under ischemic stroke. One could expect that the inhibition of autophagy could release a significant amount of free lysosomes, promoting the phagocytic clearance of dead neurons. However, autophagy inhibition is detrimental for the health and survival of microglia. The alternative autophagy stimulation strategy using rapamycin (a classical autophagy inducer drug) produces complex effects in microglia, including beneficial, neutral, and toxic effects, depending on the experimental model used for the assessment of microglial phagocytic activity and survival. Although autophagy modulation turned out to be ineffective or inconclusive for microglial phagocytosis modulation in this study, the data obtained were crucial for understanding the mechanistic complexities of lysosomal responses in microglia under ischemic stroke. Moreover, we believe that these data may eventually aid on the new development of therapeutic strategies focusing on the promotion of a microglial protective response during ischemic stroke.
More on the subject:
The art of forgetting: microglia eats memories away in mice
Modelling the new neuron-glial paradigm
Microglia and autism
References
- Beccari S, Sierra-Torre V, Valero J, Pereira-Iglesias M, García-Zaballa M, Soria FN, De las Heras-García L, Carretero-Guillén A, Capetillo-Zarate E, Domercq M, Huguet PR, Ramonet D, Osman A, Han W, Dominguez C, Faust TE, Touzani O, Pampliega O, Boya P, Schafer D, Mariño G, Canet-Soulas E, Blomgren K, Plaza-Zabala A, Sierra A (2023) Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy. Autophagy 20: 1-30. doi: 10.1080/15548627.2023.2165313 ↩
- Abiega O, Beccari S, Díaz-Aparicio I, Nadjar A, Layé S, Leyrolle Q, Gómez-Nicola D, Domercq M, Pérez-Samartín A, Sánchez-Zafra V, Paris I, Valero J, Savage JC, Hui CW, Tremblay ME, Deudero JJ, Brewster AL, Anderson AE, Zaldumbide L, Galbarriatu L, Marinas A, Vivanco Md, Matute C, Maletic-Savatic M, Encinas JM, Sierra A (2016) Neuronal hyperactivity disturbs ATP microgradients, impairs microglial motility, and reduces phagocytic receptor expression triggering apoptosis/microglial phagocytosis uncoupling. PLoS Biol 14:e1002466. doi: 10.1371/journal.pbio.1002466 ↩
1 comment
[…] versión original de este artículo apareció en inglés en Mapping Ignorance. Traducción de las […]