The cartilage produces hemoglobin to adapt to hypoxia
Author: Ramón Muñoz-Chápuli has been Professor of Animal Biology in the University of Málaga until his retirement. He has investigated for forty years in the fields of developmental biology and animal evolution.
All the cells in our body require a supply of oxygen, although in some cases, they can temporarily rely on anaerobic metabolism. Under normal conditions, cells receive this essential oxygen supply through red blood cells circulating in the bloodstream. However, oxygen doesn’t transfer directly from the red blood cell to the oxygen-consuming cell; it has to diffuse through the capillary wall and the surrounding tissue.
Certain tissues, such as muscle and the brain, have a high demand for oxygen, and diffusion from blood vessels may be insufficient to meet their needs. Skeletal muscles and the myocardium produce myoglobin, a protein very similar to hemoglobin, which imparts a red color to these organs. Another protein from the same family, discovered in 2000 and called neuroglobin, is expressed in the brain and other oxygen-sensitive tissues, such as the retina. Myoglobin and neuroglobin act as oxygen reservoirs, releasing it when necessary and protecting tissues from hypoxia.
A special case is cartilage, a tissue entirely devoid of blood vessels. Cartilage cells (chondrocytes) receive oxygen through diffusion across the cartilaginous matrix. This supply is very limited and can become insufficient when chondrocytes proliferate and hypertrophy in areas known as growth plates (Figure 1). Growth plates are located at the ends of long bones during fetal development and childhood and are essential for determining bone shape and length. It’s assumed that these oxygen-demanding areas of cartilage without vascular supply are subjected to hypoxic stress, but until now, it was not known how cartilage managed this situation.
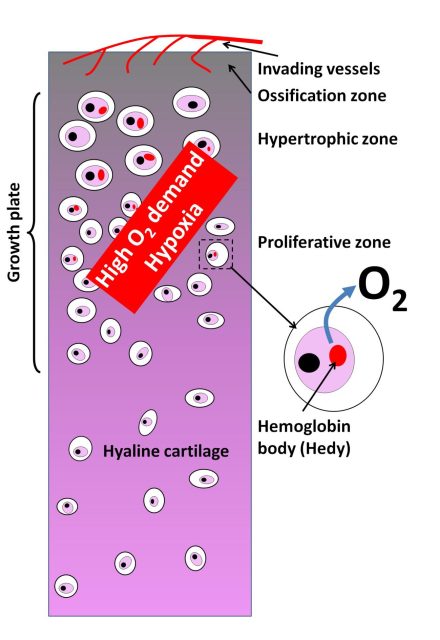
A group of chinese researchers from the Fourth Military Medical University in Xi’an, China, recently published a surprising discovery in Nature 1. Chondrocytes in the growth plates adopt a strategy similar to muscle and the brain to store and supply oxygen when needed. However, instead of developing a specific globin, chondrocytes express the same hemoglobin found in red blood cells. This is not entirely new, as traces of hemoglobin have been found in other cell types, but they were never attributed a role in oxygen homeostasis. It has been speculated that this extra-erythrocyte hemoglobin may function as an antioxidant or in iron metabolism.
The hemoglobin expressed by chondrocytes accumulates in the cytoplasm, forming a membraneless globule that the article’s authors have called “Hedy” (short for hemoglobin body). The hemoglobin in Hedy contains subunits encoded by the HBA and HBB globin genes in a 3:5 ratio. Remember that normal adult hemoglobin is typically composed of a heme group with an iron atom, two alpha chains, and two beta chains, encoded by the mentioned genes. Interestingly, Hedy formation seems to occur via phase separation, meaning it condenses from hemoglobin molecules that tend to aggregate in the cytoplasm.
Chondrocytes containing hemoglobin have slightly lower oxygen affinity compared to red blood cells, but their ability to store oxygen is crucial. In fact, researchers manipulated mice to silence hemoglobin expression in the mouse chondrocytes. As a result, they lost Hedy, experienced massive chondrocyte death in growth plates, shifted toward anaerobic metabolism (glycolysis), and the mice showed delayed skeletal development. When all HBB expression in chondrocytes was suppressed, the mice died shortly after birth, likely due to respiratory problems. Another test of their physiological role involved culturing PC12 cells, highly sensitive to oxygen deficiency, under hypoxic conditions. Both co-culturing with red blood cells and chondrocytes reversed the effects of hypoxia on these cells, demonstrating that hemoglobin from both cell types was capable of providing the necessary oxygen.
Another surprising discovery from the Chinese team was that the regulation of hemoglobin expression in chondrocytes subjected to hypoxia did not follow the classic hypoxia-inducible factor (HIF) pathway (mainly involving HIF1α). HIF1α is expressed by almost all cells in the body, its levels increase in hypoxia, but it degrades quickly in normal oxygen concentrations. When oxygen is scarce, HIF proteins stabilize and activate many genes to resist hypoxia, including angiogenic factors that promote vessel growth towards hypoxic areas and erythropoietin, which stimulates red blood cell production. It’s an energetically costly mechanism but ensures a rapid response to oxygen deficiency.
The HIF1α protein increases in chondrocytes under hypoxia, just as hemoglobin expression does. However, in mutant chondrocytes lacking HIF proteins, hemoglobin still increases in response to hypoxia. In contrast, another factor, Klf1, proved to be necessary for hemoglobin production by chondrocytes. Klf1 is essential for red blood cell development and maturation, and it’s surprising that chondrocytes have adopted it to regulate their own hemoglobin production in hypoxia, making the classic HIF1α signalling pathway unnecessary.
The authors conclude their study by posing the question whether joint pain experienced by patients with anemia or thalassemia (a hemoglobin production deficiency) could be related to a failure in oxygen homeostasis in cartilage. In any case, the production of hemoglobin by chondrocytes opens interesting and unexpected perspectives in the clinical understanding of joint pathologies.
References
- Zhang F, Zhang B, Wang Y, Jiang R, Liu J, Wei Y, Gao X, Zhu Y, Wang X, Sun M, Kang J, Liu Y, You G, Wei D, Xin J, Bao J, Wang M, Gu Y, Wang Z, Ye J, Guo S, Huang H, Sun Q. An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption. Nature. 2023 Oct 4. doi: 10.1038/s41586-023-06611-6. ↩