Simultaneous ignition of the CO oxidation on a curved platinum surface
Carbon monoxide (CO) oxidation (2CO + O2 → CO2) on platinum (Pt) group metal surfaces is the model heterogeneous gas/surface catalytic reaction. Pt itself is of the upmost importance as a catalyst for car exhaust cleaning or for the water gas shift reaction, whereas Pt crystal surfaces are model systems for investigating the catalytic CO oxidation at the atomic scale. In the last four decades many researchers have studied the separate, sequential and simultaneous interaction of CO and oxygen with Pt crystal surfaces under high or ultra-high vacuum conditions. These works have provided a good description of fundamental steps in the reaction, such as O2 dissociation, CO and O chemisorption, and CO-O interaction.
A much deeper atomic-scale insight is being gained lately through surface sensitive techniques that operate at millibar and bar pressures, such as high-pressure scanning tunneling microscopy, near-ambient pressure X-ray photoemission spectroscopy, infrared reflection absorption spectroscopy, and high-energy surface X-ray diffraction.
All these studies agree on the fundamental picture, namely, the abrupt transition at the “ignition” temperature, from the low-temperature oxidation stage, when CO covers (or “poisons”) the catalytic surface, to the high-temperature activity stage, when the CO-poisoning layer is displaced by chemisorbed oxygen, preceding the build-up, first, of two-dimensional surface oxides and, last, of bulk oxides.
At a much higher concentration of reactants, though, the CO oxidation on Pt has been shown to be much more complex than previously thought. Recent experiments undertaken at near-ambient or ambient pressure conditions have demonstrated the existence of other surface species, particularly during the active stage, which depend on the ratio of reactants and their effective transport to and from the surface. The complexity reaches its peak when low-symmetry crystal planes are considered, that is, vicinal surfaces with terrace and step sites of different atomic coordination. Vicinal planes are of major importance, since they strictly span most of the surface in spherical nanoparticles.
The very few experiments done on vicinal surfaces at millibar pressures prove the presence of structural instabilities and volatile oxides, pointing to diverse reaction mechanisms and complex structural/chemical interplay. This could be better rationalized through a simultaneous analysis of different surface planes, e.g., with cylindrical crystals.
At the millibar range, a team of researchers recently used a curved Pd(111) surface to demonstrate the structure-dependence of the CO oxidation. Planar Laser Induced Fluorescence (PLIF) experiments proved different ignition temperatures at the A- and B-type stepped sides of the sample, whereas Near Ambient Pressure X-ray Photoelectron Spectroscopy (NAP-XPS), performed under constant temperature and pressure, showed a smoothly varying distribution of poisoning and active species across the curved surface.
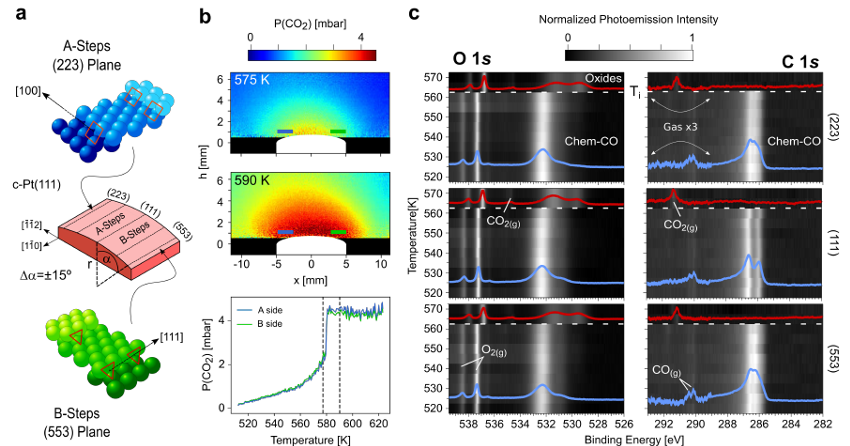
Now, the same team combines 1 PLIF and NAP-XPS to investigate the CO oxidation reaction at vicinal Pt(111) surfaces in the millibar regime, using the same curved sample design as for the Pd experiment. In contrast to the clear structure-dependence of Pd, the researchers find that the catalytic activation of Pt occurs in all vicinal planes simultaneously, irrespective of the reaction parameters.
The systematic analysis of chemical species across the entire curved surface provides the clues for this surprising behavior. As the surface CO concentration decreases when approaching ignition, minor amounts of oxygen build up at both steps and (111) terraces. First-principles theory indicates that the latter is forming a CO-Pt-O complex that binds CO molecules to terraces strongly, reducing its adsorption energy to that of low-coordinated steps, and explaining why CO abruptly desorbs at the same temperature from all crystal facets that make up the curved Pt surface. Varying the reaction conditions and the annealing rates changes the ignition temperature and the composition of the active phase, but it does not alter the simultaneous ignition process at all Pt crystal planes.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Fernando Garcia‐Martinez, Carlos García‐Fernández, Juan Pablo Simonovis, Adrian Hunt, Andrew Walter, Iradwikanari Waluyo, Florian Bertram, Lindsay R. Merte, Mikhail Shipilin, Sebastian Pfaff, Sara Blomberg, Johan Zetterberg, Johan Gustafson, Edvin Lundgren, Daniel Sánchez‐Portal, Frederik Schiller, J. Enrique Ortega (2020) Catalytic Oxidation of CO on a Curved Pt(111) Surface: Simultaneous Ignition at All Facets through a Transient CO‐O Complex Angewandte Chemie International Edition doi: 10.1002/anie.202007195 ↩