Efficient on-surface Ullmann-like reaction on poorly reactive surfaces
The way a particular reaction proceeds, described in terms of the steps involved, is called mechanism. The study of organic chemistry is, to a great extent, the study of reaction mechanisms and textbooks content both their description and their applications. But something has come to revolutionize the world of mechanisms: surface chemistry. On-surface synthesis is appearing as an extremely promising research field aimed at creating new organic materials. A large number of chemical reactions have been successfully demonstrated to take place directly on surfaces through unusual reaction mechanisms.
On-surface synthesis is a newly developing field of research that aims at making use of well-defined solid surfaces as confinement templates to initiate chemical reactions. The concepts of supramolecular chemistry are applied so that surfaces can be functionalized in such a way that nanometer-sized elementary building-blocks can self-assemble on them. Therefore, on-surface synthesis represents in a sense an extension of heterogeneous catalysis whereby the initial precursors, the intermediate states, and the reaction products all remain in an adsorbed state, usually in the submonolayer regime.
The interest in creating nanoarchitectures directly on surfaces is threefold. First, on-surface synthesis gives access to new reaction mechanisms in mild conditions that would be not easily accessible in standard chemistry conditions. Second, it represents an efficient route to the formation of robust organic networks and 2D polymers. And finally, the full range of available surface science techniques can deliver exquisite characterization of the different reaction processes with atomic precision.
Throughout the past decade, on-surface chemistry has proven to be an extraordinary tool for building sp2 bond-based carbon nanostructures with unprecedented atomic precision. Motivated by the fact that such carbon structures, which are hard to synthesize by common wet chemistry methods, have prominent electronic properties deserving implementation into electronic devices, the interest of exploring surface-induced molecular reactions has grown exponentially.
The Ullmann coupling reaction, in conjunction with a subsequent cyclodehydrogenation step, is recognized as the most promising pathway toward designing carbon-based nanostructures for molecular electronics on surfaces. However, bringing such materials into nanodevices remains a challenge, as the surface-assisted Ullmann reaction is mostly employed in ultrahigh vacuum (UHV) conditions on single-crystal noble metal substrates (gold, silver, and copper) given their catalytic activity. Nevertheless, these metal-adsorbed nanostructures present limitations since they remain electronically coupled to the underlying catalyzing substrate. Thus, the decoupling of the synthesized nanostructures must be achieved afterward for their implementation into devices, which is currently performed by cumbersome postgrowth transfer methods.
The bottom-up synthesis of carbon-based nanomaterials directly on semiconductor surfaces allows for the decoupling of their electronic and magnetic properties from the substrates. However, the typically reduced reactivity of such nonmetallic surfaces adversely affects the course of these reactions.
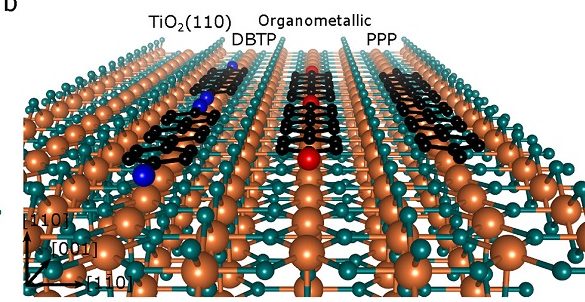
Now, a team of researchers achieve 1 a high polymerization yield (it is practically tripled) of halogenated polyphenyl molecular building blocks on the semiconducting TiO2(110) surface via concomitant surface decoration with cobalt atoms, which catalyze the Ullmann coupling reaction.
Specifically, cobalt atoms trigger the debromination of 4,4″-dibromo-p-terphenyl molecules on TiO2(110) and mediate the formation of an intermediate organometallic phase already at room temperature. As the debromination temperature is drastically reduced (lowered by 55 K), homocoupling and polymerization readily proceed, preventing presursor desorption from the substrate and entailing a drastic increase of the poly-para-phenylene polymerization yield.
The researchers show that the general efficacy of this mechanism by means of an iodinated terphenyl derivative, which exhibits similar dehalogenation and reaction yield.
This study presents a new strategy for implementing the on-surface Ullmann-like reaction with high efficiency on poorly reactive semiconducting or insulating surfaces such as TiO2(110), opening a promising avenue for synthesizing graphene-based nanostructures, such as graphene nanoribbons and nanoporous graphene structures, directly on more technologically relevant surfaces.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Mikel Abadia, Ignacio Piquero-Zulaica, Jens Brede, Alberto Verdini, Luca Floreano, Johannes V. Barth, Jorge Lobo-Checa, Martina Corso, and Celia Rogero (2024) Enhancing Haloarene Coupling Reaction Efficiency on an Oxide Surface by Metal Atom Addition Nano Lett. doi: 10.1021/acs.nanolett.3c04111 ↩