The role of copper in the Ullmann reaction
Organic chemistry uses lots and lots of names. Not only purely chemical names, but people’s names. This is so because the field is so broad that you need to name not only substances, that usually have two names, the official – from IUPAC rules – and the nickname, usually much shorter and easier to remember (and pronounce), but also processes, reactions and collections of all things organic.
Names are so important in organic chemistry that it was the base of a detective short story by Isaac Asimov, himself a biochemist, called What’s in a name? where the murderer is identified when she does not recognise a name that no organic-chemistry-related person would have failed to do, Beilstein, from Beilstein’s Handbook of Organic Chemistry. If Asimov would have chosen as the setting for his story a factory instead of an university, he could have used another name that is recognised at once: Ullmann. In this case, from Ullmann’s Encyclopedia of Industrial Chemistry.
But Fritz Ullmann an his collaborators (more about on of them at the end) also named some reactions and processes more than a century ago. One of them has attracted a lot of attention recently.
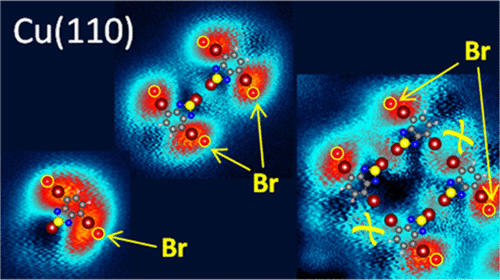
Discovered by Fritz Ullmann and Bielecki in 1901 by adding copper particles to a solution containing bromine derivatives of benzene, the Ullmann reaction is undergoing a considerable revival in the past decade. Its application allowing the reliable synthesis of extended polymers has opened a fruitful path in surface chemistry, leading to the formation of polyphenyls, graphene structures of well-defined shape, or covalently assembled structures with engineered electronic and functional properties. We have seen examples of these applications here, here or here.
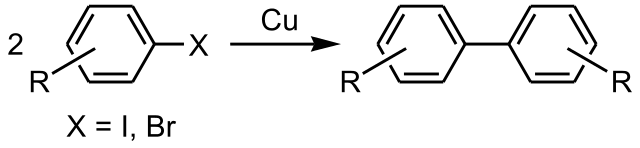
The Ullmann reaction is the metal-catalyzed coupling of halogene-benzene derivatives leading to biaryls (an aryl group is a group obtained by removing a hydrogen atom from and aromatic compound; if the aromatic compound is benzene, the aryl is the phenyl group) and larger carbon-based structures. This reaction offers an unprecedented opportunity to access the molecular functionality by improving the mechanical stability and electron conductance, which are essential to advance in the realization of organic-based electronics.
Catalysts, like copper, provide an alternative pathway by which the reaction can proceed, in which the activation energy is lower. It thus increases the rate at which the reaction comes to equilibrium. The catalyst itself takes part in the reaction without undergoing any permanent chemical change, although it may undergo a physical one. In the classical Ullmann reaction, the oxidation of copper with the formation of molecular cuprate intermediates and copper halides as side reaction products precedes the cross-coupling reaction. Whereas there is general agreement that at a certain point of the reaction a copper-coordinated structure is formed, the debromination mechanism, which is the rate-limiting step of the reaction, has been scarcely investigated. At this point, it is not clear whether the formation of radicals or organo-copper complexes or the oxidative addition process precedes the formation of the biaryl.
How can we get to know what is really happening? We could make some calculations and then design some clever experiments measuring minute amounts of energy variations to discriminate between them. But this has two problems: the first one is that, in the best-case scenario, the margin of error in the results would be of the same order of magnitude of the variations in energy measured; the second is that a significant result, if any, would be a correlation and, therefore, non-conclusive. It is much better to look at what is really happening. Quite literally. Today, we can visualize what is happening at the copper surface using scanning probe techniques and X-ray photoemission spectroscopy to monitor the oxidation state of carbon and bromine atoms.
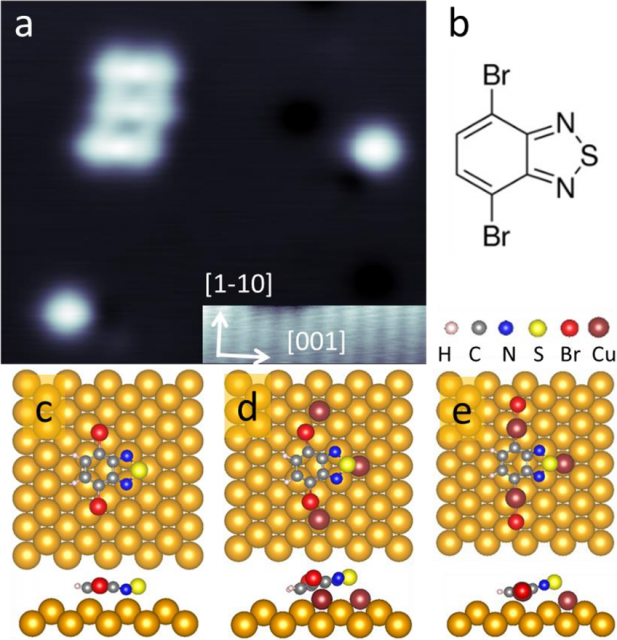
Ane Sarasola (UPV/EHU & DIPC), Ana Barragán (UPV/EHU & CFM) and Ikerbasque Research Professor Lucia Vitali (UPV/EHU & CFM) have addressed 1this question by characterizing the molecular debromination reaction of a prototypical molecule, namely, 4,7-dibromobenzo[c]-1,2,5-thiadiazole (nicknamed 2Br-BTD) adsorbed on Cu(110). They use scanning tunneling probe techniques (STM/STS) and density functional theory (DFT) calculations.
The researchers demonstrate that the oxidative addition of Cu atoms on the crystal surface leads to the formation of a −C−Cu−Br structure, i.e., a mechanistic sequence for the Br scission that is what is expected to precede the Ullmann coupling reaction. Besides, they show that the debromination process requires the concerted action of neighbouring precursors. This mechanism explains the debated key role, from a kinetic point of view, of the Cu atom in the debromination reaction.
On a side note, let’s remember that next monday, February 11, is the International Day of Women and Girls in Science, we can put some historical perspective on this work:
The findings of these three female researchers make justice to those of a pioneer, Irma Goldberg, Ullmann’s wife and excellent organic chemist, who published a paper in 1904 on using copper as a catalyst for the preparation of a phenyl derivative of thiosalicylic acid, and you guessed it, using the Ullmann reaction. This work introduced great improvements to previous forms of the method, and was extremely helpful for laboratory-scale preparations. She coordinated on other forms of chemistry research with her husband, Fritz Ullmann, in what they called the Ullmann-Goldberg collaborative. And, as we started talking about names, let’s mention that Irma Golberg was the first female that gave name to an organic reaction 2: the Goldberg reaction, in 1906.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
References
- Ane Sarasola, Ana Barragán and Lucia Vitali (2018) Cooperative Action for Molecular Debromination Reaction on Cu(110) J.A.C.S. doi: 10.1021/jacs.8b10329 ↩
- Olson, J. A., & Shea, K. M. (2011) Critical Perspective: Named Reactions Discovered and Developed by Women. Accounts of Chemical Research, 44(5), 311–321.doi:10.1021/ar100114m ↩
1 comment
[…] Etorkizuneko elektronika, aldaera guztietan, eskala industrialean material egokiak modu efizientean lortzearen araberakoa izango da. Grafenoan eta bere aldaeretan oinarritutako elektronikaren kasuan, lehengai oirganikoetaik ekoiztu beharko dira eta, horretarako, haien sintesi eta katalisi erreakzioen ezagutza ezinbestekoa da. DIPCkoek aurrerapausua eman […]