Charge transfer and symmetry affect π-magnetic nanostructures
Whenever an electric current flows a magnetic field is produced; as the orbital motion and the spin of atomic electrons are equivalent to tiny current loops, individual atoms create magnetic fields around them, when their orbital electrons have a net magnetic moment as a result of their angular momentum. The magnetic moment of an atom is the vector sum of the magnetic moments of the orbital motions and the spins of all the electrons in the atom. The macroscopic magnetic properties of a substance arise from the magnetic moments of its component atoms and molecules. Interestingly, the mechanistic origins of magnetism in materials can also be viewed as breaks in symmetry that allow for unpaired electrons.
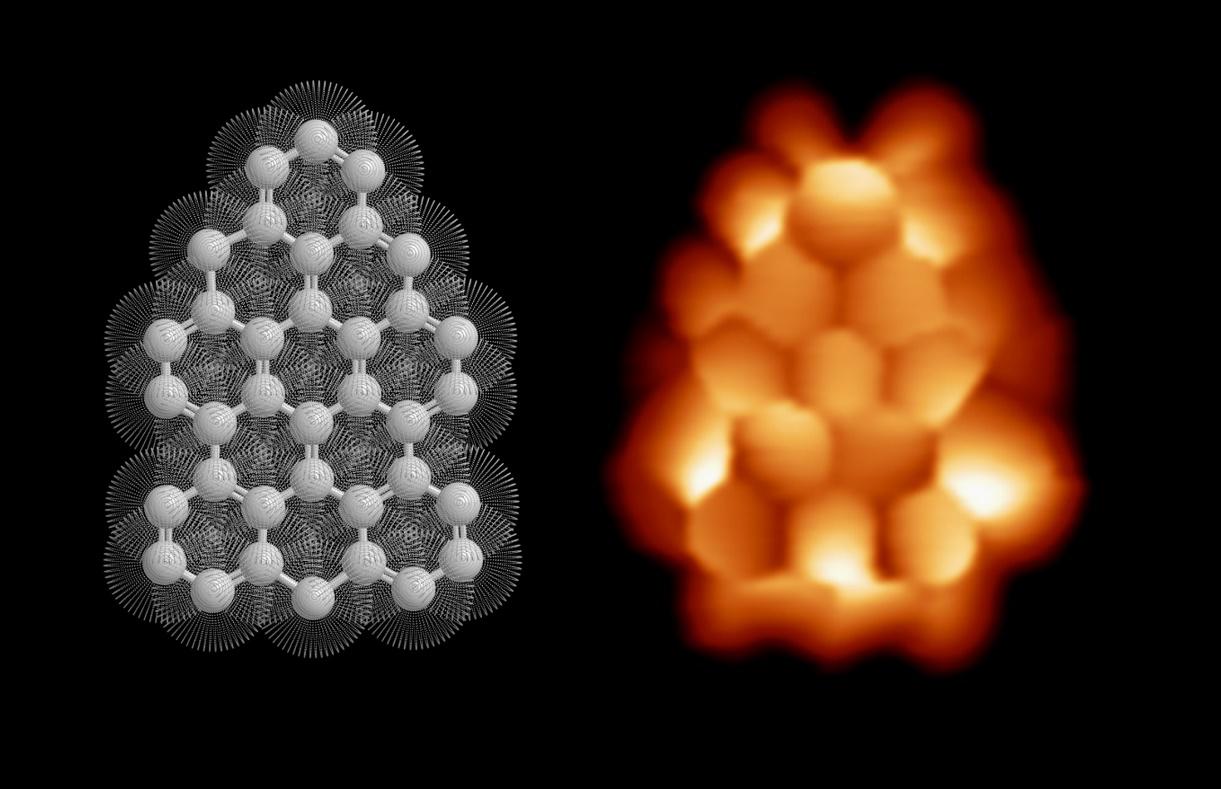
Though long-time associated with d- and f-shell electrons of metals, unpaired electrons can also emerge in p-shell electrons of carbon-based materials, conferring magnetic properties to π-conjugated structures. Thus, engineering π-magnetism of carbon nanomaterials requires a precise control of the most trivial changes in the chemical structure. In this regard, on-surface synthesis – simultaneously affording atomic precision, inert environment, and substrate stabilization – has proven effective, allowing access to a myriad of π-magnetic molecular materials such as triangulenes, graphene nanoribbons, and other graphene nanofragments.
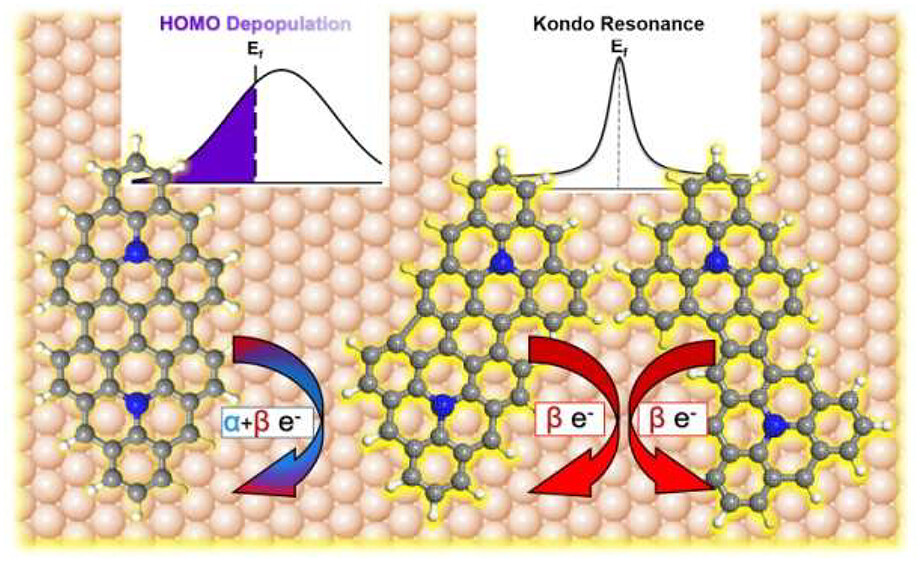
Aside from their intrinsic magnetic properties, charge transfer between carbon nanostructures and a metal surface can also generate (or modify) π-magnetism. Adding/removing electron(s) to/from a molecule may modify the spin balance. This requires spin-selective charge transfer: the number of α and β spin electrons transferring to and from the surface must be different. Otherwise, equal contributions would not change the net spin, as in conventional charge transfer scenarios resulting in the depopulation of frontier molecular orbitals. Although each case has been widely reported, a better understanding of parameters influencing the emergence of magnetism vs conventional orbital depopulation by charge transfer could allow their rational tuning.
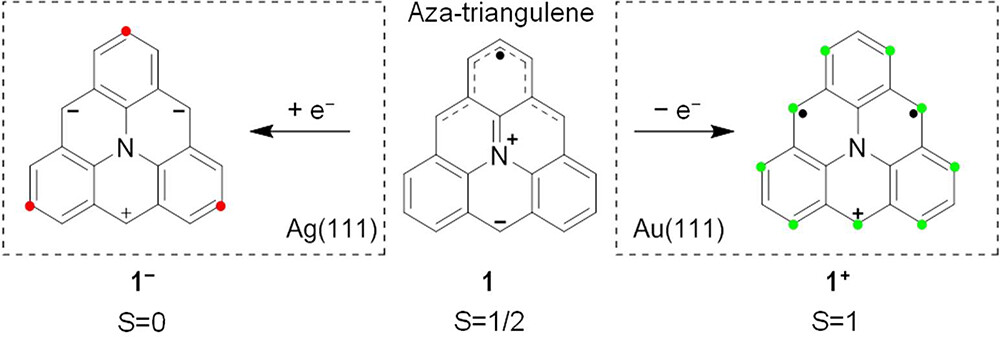
Triangulene is the most studied molecule displaying π-magnetism due to sublattice imbalance. Inspired by how heteroatom doping enriches the chemistry and physical properties of nanographenes, a team of researchers recently showed 1 that the presence of a nitrogen atom in the carbon framework of triangulene, as in aza-triangulene, triggered charge transfer in opposite directions on Au(111) and Ag(111). Because the redox potentials of 1 (see Scheme 1) lie in the narrow window between the work functions of these substrates, an oxidized cationic species 1+ with open-shell S = 1 character was observed on Au(111) while a reduced closed-shell anionic species 1– was observed on Ag(111). Therefore, 1, in particular upon fusion into larger derivatives, offers an ideal platform to systematically investigate parameters influencing the spin relevance of charge transfer to and from metal surfaces, as associated with magnetic properties.
Now, the team has fabricated 2 various extended triangulene structures by the fusion of 1 and investigated their tunable magnetic properties. By using high-resolution bond-resolving scanning tunneling microscopy (BR-STM) with a CO-functionalized STM tip, the researchers show that coupling products are random on Ag(111), mostly limited by steric effects, and present closed-shell character.
In contrast, on Au(111), fused dimeric products resulting from straight and staggered alignment of reactive carbon sites (those with the highest spin density) were observed , producing symmetric and asymmetric structures, respectively.
Scanning tunneling spectroscopy (STS), coupled with density functional theory (DFT) calculations, reveals that the closed-shell structure of aza-triangulene on Ag(111) leads to closed-shell dimers covalently coupled through sterically accessible carbon atoms. Meanwhile, its open-shell structure on Au(111) leads to coupling via atoms displaying a high spin density, resulting in symmetric or asymmetric products. Interestingly, whereas all dimers on Au(111) exhibit similar charge transfer properties, only asymmetric ones show magnetic fingerprints due to spin-selective charge transfer.
These results expose clear relationships among molecular symmetry, charge transfer, and spin states of π-conjugated carbon-based nanostructures. Hence, charge transfer and symmetry considerations could synergistically affect magnetic properties toward the future design of π-magnetic nanostructures.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Tao Wang, Alejandro Berdonces-Layunta, Niklas Friedrich, Manuel Vilas-Varela, Jan Patrick Calupitan, Jose Ignacio Pascual, Diego Peña, David Casanova, Martina Corso, and Dimas G. de Oteyza (2022) Aza-Triangulene: On-Surface Synthesis and Electronic and Magnetic Properties J. Am. Chem. Soc. doi:10.1021/jacs.1c12618 ↩
- Jan Patrick Calupitan, Alejandro Berdonces-Layunta, Fernando Aguilar-Galindo, Manuel Vilas-Varela, Diego Peña, David Casanova, Martina Corso, Dimas G. de Oteyza, and Tao Wang (2023) Emergence of π-Magnetism in Fused Aza-Triangulenes: Symmetry and Charge Transfer Effects Nano Lett. doi: 10.1021/acs.nanolett.3c02586 ↩