The secret structures of benzodithiophene polymers unlock solar energy
The secret structures of benzodithiophene polymers unlock solar energy
Organic electronics in the form of organic solar cells — thin, flexible, and printable on everyday surfaces — promise a sustainable future in renewable energy. Unlike traditional silicon-based solar panels, organic photovoltaic (OPV) devices rely on carbon-based polymers, a class of molecules more commonly associated with plastic bags than power generation. Among these, a family of materials built around a chemical unit called benzodithiophene (BDT) has emerged as a star performer.
In a landmark study, a team of researchers has revealed the hidden structural complexities of these BDT-based polymers. Their findings 1 provide a new blueprint for how these materials pack and behave in solid form — a critical factor that determines how well they can harvest sunlight and convert it into electricity.
Why structure matters in organic electronics
At the heart of every organic solar cell lies a thin film made of semiconducting polymers. These polymers absorb sunlight and convert it into mobile electrical charges. However, this process hinges on the microscopic arrangement of polymer chains. Unlike silicon, where atoms are arranged in a tidy crystalline lattice, polymer chains are long, floppy, and chemically diverse. How these chains pack together — whether in orderly rows or messy tangles — greatly influences how efficiently charges move through the material.
Until now, researchers largely relied on two classical models to understand polymer structure: amorphous (completely disordered) and semicrystalline (partially ordered). But these models fall short for semiconducting polymers like the BDT-based family. These materials display a complex mixture of order and disorder that defies traditional classification.
Uncovering a hidden mesophase
The team explored three high-performance BDT polymers: D18, PBDB-T-Cl, and PBnDT-FTAZ. They used a battery of advanced techniques to probe the solid films these polymers form. These included atomic force microscopy (AFM), to visualize surface topography and nanoscale features; grazing-incidence X-ray scattering (GIWAXS), to probe molecular packing and orientation within the films; and fast scanning calorimetry (FSC), to study thermal transitions and phase changes.
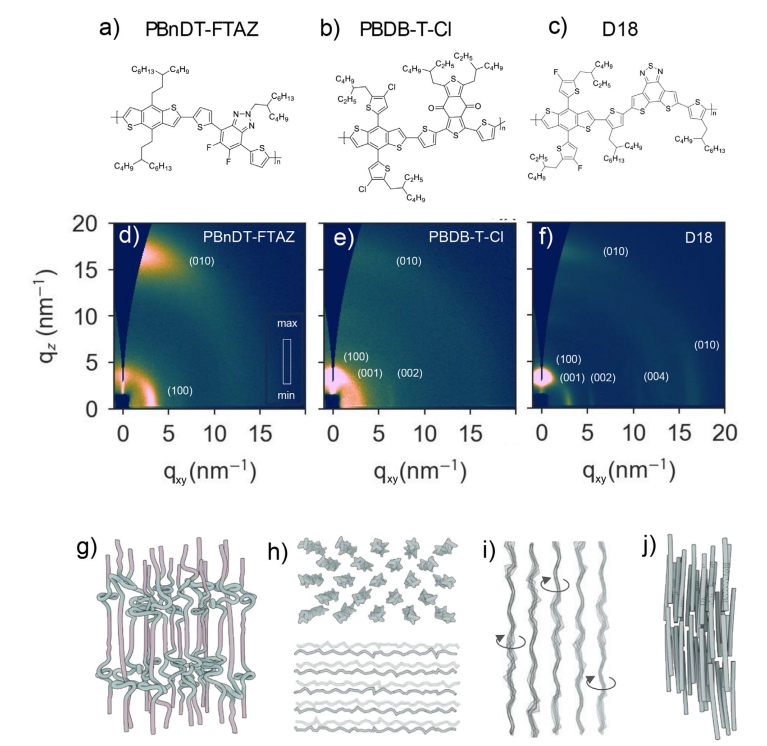
What they found was startling: these polymers do not fit neatly into the “amorphous” or “semicrystalline” categories. Instead, they form what’s known as a solid mesophase — a state of matter somewhere between a crystal and a glass. In this state, the polymers assemble into layered structures, with ordered “plates” of rigid, π-conjugated backbones, aromatic molecular units in the polymer that conduct electricity due to delocalized electrons, stacked between more disordered regions dominated by floppy, aliphatic, flexible hydrocarbon chains that provide structural flexibility but are non-conductive, side chains.
This alternating pattern of order and disorder gives rise to lamellar (layered) structures a few nanometres thick. Yet, unlike fully crystalline materials, these layers are riddled with imperfections. Diffraction studies revealed that these stacks exhibit long-range periodicity in some directions — enough to qualify as organized — but lack the three-dimensional symmetry of true crystals.
Fibrils and domains: A biphasic microstructure
On a larger scale, the researchers observed that these layered regions aggregate into nanoscale fibrils, fibre-like structures which align along specific directions during processing. For example, rubbing the surface of a D18 film at high temperature caused the fibrils to align with the rubbing direction. Subsequent optical and X-ray measurements confirmed that the polymer backbones also aligned within the fibrils, a desirable feature for efficient charge transport in solar cells.
Importantly, these ordered fibrils coexist with more disordered, glass-like regions. These disordered domains lack the spatial periodicity of the mesophase and behave more like traditional polymer glasses. The resulting biphasic microstructure — comprising both mesophase fibrils and amorphous regions — is key to understanding how these materials perform in real devices.
High-performance polymers like D18 showed a high degree of mesophase ordering and minimal glassy content, while others like PBnDT-FTAZ contained more disordered regions. This structural difference helps explain their relative efficiency in OPV devices.
How temperature changes everything
Another critical insight from the study involves the materials’ response to heat. Using FSC, the researchers tracked how the structure of each polymer evolved with temperature. They discovered that the transition from ordered mesophase to disordered melt occurred in a single, sharp step — a hallmark of a thermotropic transition. In other words, a phase change induced by temperature where the layers collapsed and the material began to flow at the same temperature, suggesting a unique coupling between molecular order and phase change.
Interestingly, the nature of this transition varied among the polymers. Some, like PBDB-T-Cl and D18, exhibited a two-phase behavior with distinct low- and high-temperature regions. Others, like PBnDT-FTAZ, showed three-phase behaviour with an intermediate phase, likely a less ordered precursor to the mesophase.
This nuanced thermotropic behaviour explains why certain thermal treatments — like annealing films at specific temperatures — can dramatically improve device performance. By tuning the annealing temperature, researchers can optimize the balance between order and disorder, enhancing the pathways through which charges flow.
Implications for organic electronics
Understanding the microstructure of semiconducting polymers is more than an academic exercise. It’s a crucial step in designing better materials for a range of organic electronics — not just solar cells but also transistors, light-emitting diodes, and bioelectronic devices. The discovery of this unique solid mesophase in BDT polymers offers a new paradigm for molecular engineering.
Rather than seeking perfect crystals or settling for amorphous networks, researchers can now aim to manipulate these intermediate mesophases. By controlling factors like side-chain length, backbone rigidity, and processing temperature, it may be possible to “dial in” the optimal balance of order and mobility.
This study also underscores the importance of viewing these polymers not as homogeneous materials, but as complex, phase-separated systems. The coexistence of mesophase and glassy regions, and their sensitivity to thermal history, highlights the need for precise control over film formation during device fabrication.
A blueprint for the future
The researchers have given the scientific community more than just a snapshot of polymer structure — they’ve provided a framework for understanding how structure, morphology, and processing interact in organic electronics. Their work bridges chemistry, physics, and materials science, offering a roadmap for designing next-generation semiconducting polymers.
As the world seeks sustainable energy solutions, insights like these will be essential. After all, turning sunlight into electricity efficiently and cheaply may one day depend not just on what molecules we make, but on how those molecules choose to dance together when the lights come on.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Matteo Sanviti, Sara Marina, Xabier Rodriguez-Martínez, Jesika Asatryan, Valerio Di Lisio, Sandra Hultmark, Junkal Gutierrez, Eduardo Solano, Jeromy J. Rech, Eugenio L. Solla, Wei You, Agnieszka Tercjak, M. Eugenio Vázquez, Daniele Cangialosi, Christian Müller, Harald Ade, and Jaime Martin (2025) Decoding the Structure of Benzodithiophene Polymers for High-Efficiency Organic Solar Cells Advanced Functional Materials doi: 10.1002/adfm.202503634?af=R ↩