How tiny particles navigate tumours: Understanding nanoparticle penetration in 3D cell models
How tiny particles navigate tumours: Understanding nanoparticle penetration in 3D cell models
Nanoparticles, those minuscule marvels measured in billionths of a metre, hold immense promise for revolutionizing cancer treatment. Their ability to deliver drugs directly to tumours could transform therapies, but only if they can navigate the dense, complex environment of tumour tissue. A new review 1, that includes a new experimental data set, explores this challenge, examining how nanoparticle size influences their ability to penetrate 3D tumour spheroids—simplified models that mimic real tumours. By testing gold nanoparticles and using sophisticated imaging techniques, the study reveals critical insights into designing nanoparticles for effective cancer therapy. This article breaks down the findings, exploring why size matters, how spheroids serve as testing grounds, and what lies ahead for nanoparticle research.
Why size matters
Imagine trying to water a plant through a mesh screen: smaller droplets slip through easily, while larger ones get stuck. Similarly, the size of nanoparticles determines how well they can infiltrate the tightly packed cells of a tumour. The study tested gold nanoparticles (AuNPs) ranging from 5 to 100 nanometres in diameter, finding that smaller particles, like those around 5 nm, penetrate deeper into tumour spheroids. Larger nanoparticles, such as those at 50 or 100 nm, often get trapped near the surface, limited by the dense cellular structure. This size-dependent behaviour is crucial because deeper penetration means drugs can reach more cancer cells, improving treatment effectiveness. Understanding these dynamics helps researchers design nanoparticles that can slip through tumour barriers like water through a fine mesh.
The role of 3D tumour spheroids
To study nanoparticle penetration, researchers need models that mimic tumours without the complexity of living systems. Enter 3D tumour spheroids, which are like simplified architectural models of a city—capturing key features of tumour tissue but lacking the full intricacy of real tumours. These spheroids, made from MCF-7 breast cancer cells, are tiny spheres (less than 0.52 mm³) that replicate the dense, multilayered structure of tumours. Unlike flat, 2D cell cultures, spheroids better mimic the physical barriers nanoparticles face, such as tight cell junctions and limited diffusion. However, they fall short of real tumours, which are much larger (50–100 mm³) and include stromal components like fibroblasts and immune cells. To bridge this gap, researchers use spheroids as a practical starting point to test how nanoparticles navigate tumour-like environments, setting the stage for more advanced studies.
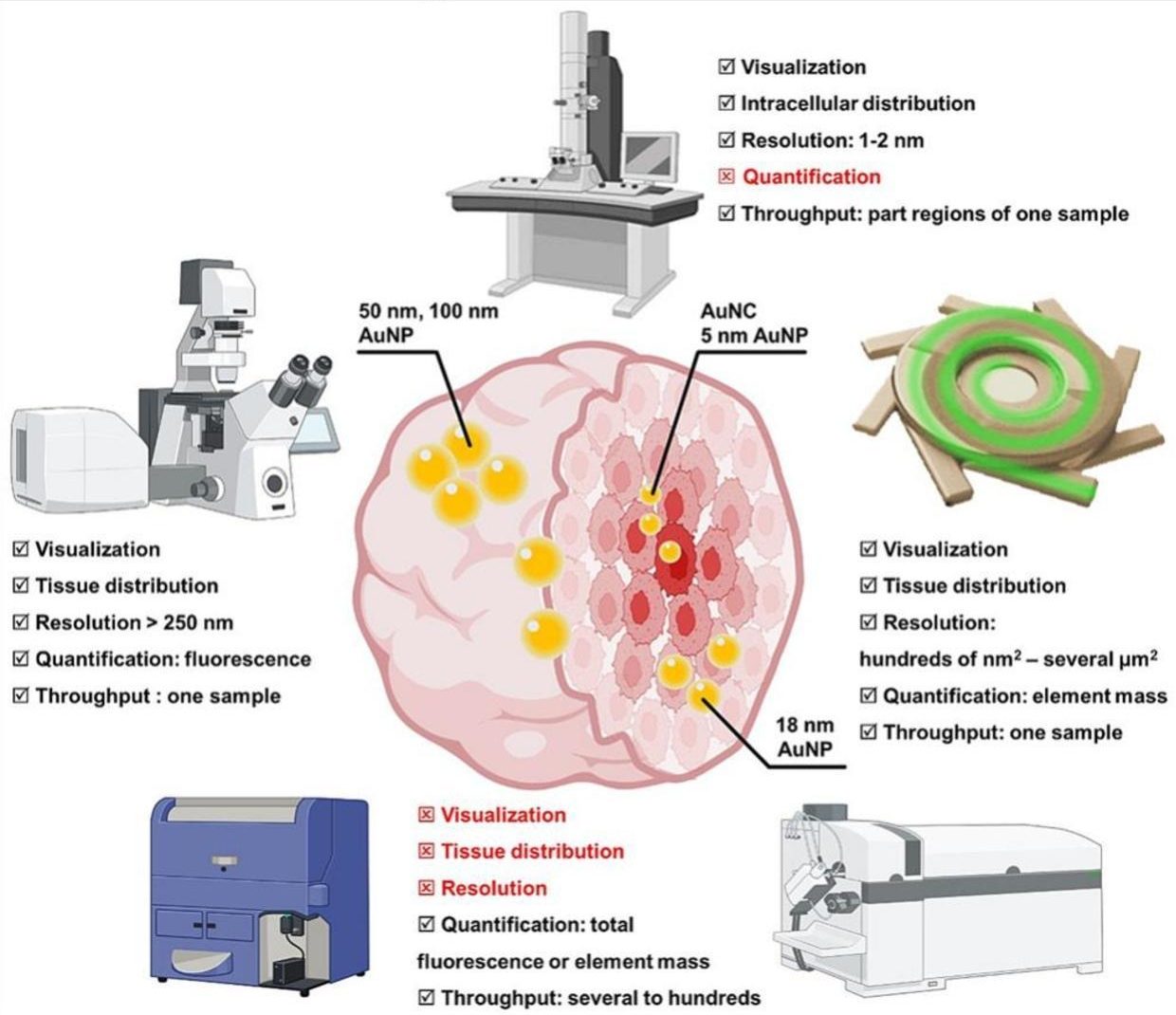
Putting nanoparticles to the test
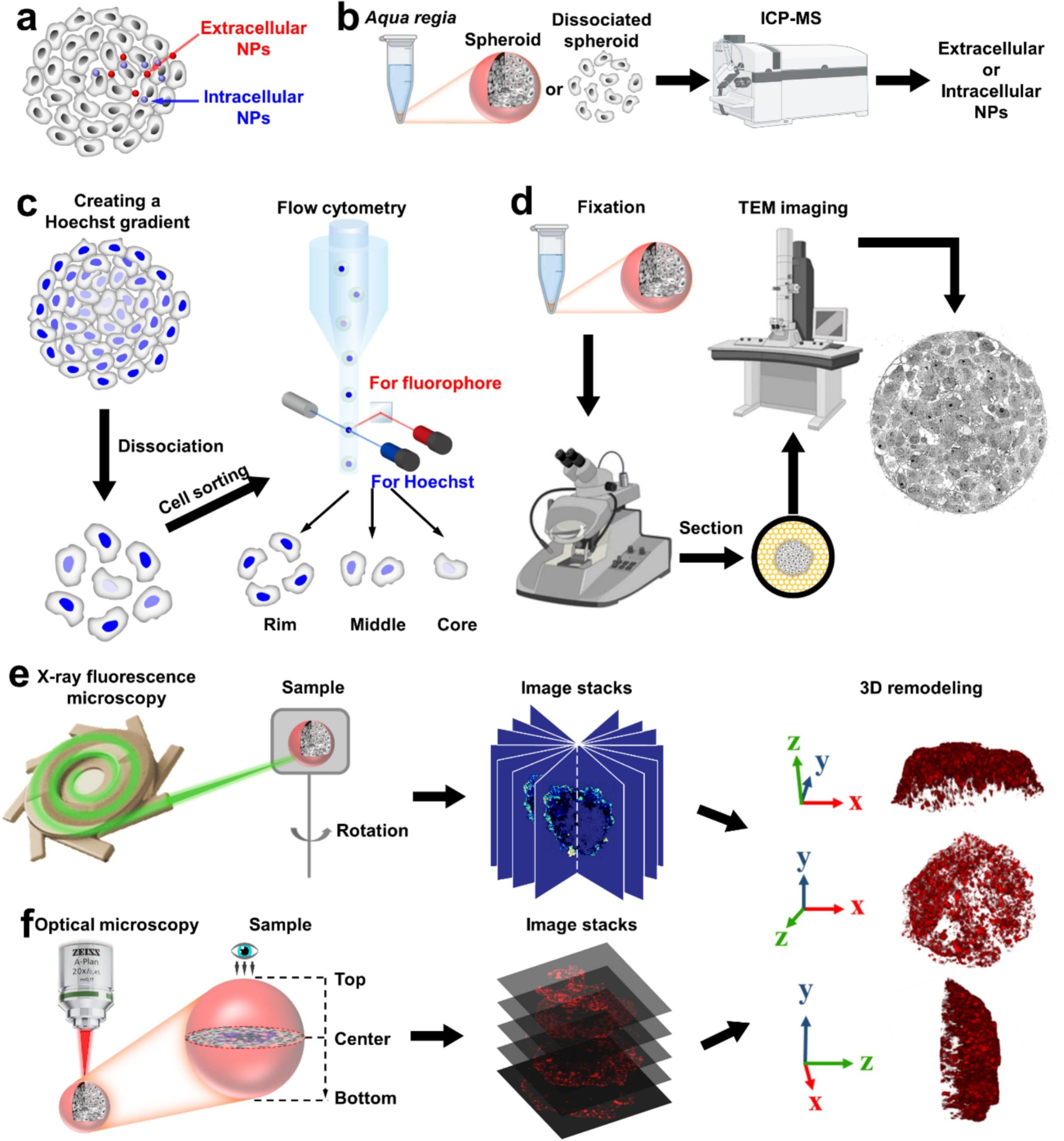
To explore how nanoparticle size influences penetration, the researchers tested gold nanoparticles of 5, 50, and 100 nanometres within MCF-7 breast cancer spheroids, using bismuth nanoparticles (37 nm) as a control to normalize experimental variability. They employed a suite of advanced techniques to track the nanoparticles’ journey, each offering unique insights into their distribution and depth. Inductively coupled plasma-mass spectrometry precisely quantified the amount of gold within the spheroids, revealing total nanoparticle uptake with high accuracy. Fluorescence microscopy illuminated fluorescently labelled nanoparticles, providing a three-dimensional view of their spread, though light scattering in dense tissue sometimes limited clarity. X-ray fluorescence imaging mapped the distribution of gold and bismuth elements without needing fluorescent labels, despite requiring high-energy X-rays and being time intensive. Transmission electron microscopy offered high-resolution images, pinpointing whether nanoparticles were inside or outside cells, though it was limited to thin sample slices. By combining these methods, the researchers gained a comprehensive understanding of how nanoparticle size affects penetration, with each technique contributing a piece to the puzzle despite its specific strengths and limitations.
Beyond size: Other factors at play
While size is a key factor, it’s not the whole story. The study highlights other influences on nanoparticle penetration, such as surface chemistry, which can affect how particles interact with cells, and the tumour microenvironment, including factors like cell density and extracellular matrix composition. Spheroids, while useful, don’t fully capture the complexity of real tumours, which include blood vessels, immune cells, and stromal components that can alter nanoparticle behaviour. For example, the absence of blood flow in spheroids means they can’t replicate how nanoparticles might travel through a tumour’s vascular network. These limitations underscore the need for more advanced models to translate lab findings to clinical success, ensuring nanoparticles can navigate the full complexity of a tumour’s terrain.
What’s next?
The insights from this study pave the way for smarter nanoparticle design, but there’s still work to be done. Advanced models like organoids, which incorporate multiple cell types, or tumours-on-a-chip, which simulate blood flow and tissue dynamics, could provide a more realistic testing ground. The study also suggests that immune cells might play a role in nanoparticle transport, potentially acting as carriers to deliver therapeutics deeper into tumours. By refining nanoparticle properties and testing them in these sophisticated systems, researchers aim to bridge the gap between lab experiments and real-world treatments. These advancements could lead to therapies that target cancer with unprecedented precision, overcoming the barriers that have long challenged drug delivery.
Nanoparticles hold immense promise for revolutionizing cancer therapy, but their success hinges on their ability to navigate the complex terrain of tumours. Just as water must reach a plant’s roots to nourish it, nanoparticles must penetrate deep into tumour tissue to deliver their therapeutic payload. Studies like this one, which reveal how size influences penetration in 3D tumour spheroids, bridge the gap between laboratory discoveries and clinical applications. By refining nanoparticle design and leveraging advanced models like organoids or tumours-on-a-chip, researchers are paving the way for treatments that could one day target cancer with unprecedented precision, offering hope for more effective therapies.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Dingcheng Zhu, Dennis Brückner, Martin Sosniok, Marvin Skiba, Neus Feliu, Marta Gallego, Yang Liu, Florian Schulz, Gerald Falkenberg, Wolfgang J. Parak, Carlos Sanchez-Cano (2025) Size-dependent penetration depth of colloidal nanoparticles into cell spheroids Advanced Drug Delivery Reviews doi: 10.1016/j.addr.2025.115593. ↩