Investigating the fundamental stages of the CO oxidation reaction using a kinked Pt crystal
Investigating the fundamental stages of the CO oxidation reaction using a kinked Pt crystal
Carbon monoxide (CO) oxidation (2CO + O2 → CO2) on platinum (Pt) group metal surfaces is the model heterogeneous gas/surface catalytic reaction. Pt itself is of the upmost importance as a catalyst for car exhaust cleaning or for the water gas shift reaction, whereas Pt crystal surfaces are model systems for investigating the catalytic CO oxidation at the atomic scale. In the last four decades many researchers have studied the separate, sequential and simultaneous interaction of CO and oxygen with Pt crystal surfaces under high or ultra-high vacuum conditions. These works have provided a good description of fundamental steps in the reaction, such as O2 dissociation, CO and O chemisorption, and CO-O interaction.
However, most investigations to date have occurred under controlled conditions, using single crystals or in ultrahigh vacuum environments, detached from the real conditions of industrial applications. Consequently, extending surface science findings to real catalytic systems, operating at atmospheric pressures (pressure gap), and involving powder/nanoparticle catalysts (materials gap), may lead to inaccuracies.
New experimental approaches are needed to allow the most powerful techniques to bridge both the pressure gap, such as near ambient pressure x-ray photoemission (NAP-XPS), and the materials gap through novel sample designs. In the latter case, it is important to realize that metallic nanoparticles possess multiple facets, making it difficult to track their specific activity and interactions during the chemical reaction. Therefore, conventional single-crystal surfaces offer limited information as they represent only one plane, failing to mirror the complex, multifaceted structure of actual catalysts.
One potential approach to bridge the structural gap involves the utilization of cylindrical sectors of single crystals. Their curved surfaces allow for a smooth transition in the crystal orientation, enabling a systematic comparison of various facets under identical reaction conditions. With a proper selection of the crystal sector, a curved surface also offers a sophisticated but consistent means of analyzing vicinal surfaces, hence the effect of undercoordinated, step atoms in surface-catalyzed reactions.
The combined use of curved surfaces with (NAP-)XPS has successfully demonstrated its potential to straightforwardly assess the role of steps in the CO oxidation on Pd, Pt, and Rh vicinals or Ag-oxidation, among other matters. The curved geometry allowed the identification of surface species at different reaction stages and an accurate determination of the ignition temperature across different facets. Surprisingly, species and ignition temperatures were found the same at A-type ({100}-oriented microfacets) and B-type ({111} microfacets) stepped Pt(111) surfaces, by contrast to the expected A/B asymmetries observed in Pd and Rh. Yet the question arises whether such homogeneous and symmetric behavior in Pt occurs far beyond the (111) plane or features different step geometries in the vicinity of the Pt(111) surface, such as the more open kinked steps. Their complexity is probably the reason why they have been poorly investigated with XPS in the CO oxidation context, even under UHV conditions.
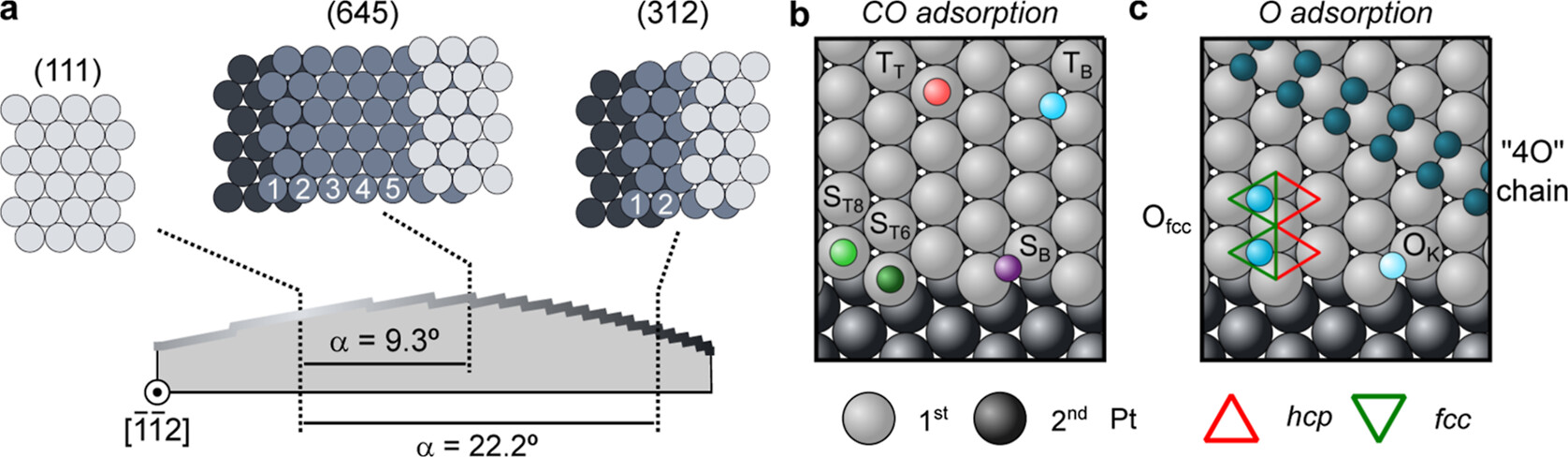
Now, a team of researchers, using a Pt curved crystal with a special design and UHV-XPS, investigates 1 a wide range of Pt vicinal surfaces with kinked steps during CO and O saturation at 300 K. They represent the two fundamental stages of the CO oxidation reaction, i.e., the CO-poisoned and the O-active surfaces.
The sample is a cylindrical sector of a Pt crystal. The reference (111) plane is located close to one edge, allowing Pt vicinal surfaces to be spanned with kinked steps up to a α = 28° vicinal (or tilt) angle with respect to the (111) plane. The large vicinal angle range allows the team to reach all vicinal surfaces from the (111) beyond the densely kinked (312) surface (2-atom-wide terraces) at the opposite sample edge.
Through uptake and saturation experiments on this sample, the researchers identify a variety of CO and O species, which can also be quantified and discussed in model adsorbate structures, thanks to the systematic approach that only the curved geometry allows.
In the CO-saturated case, a preferential adsorption at step edges is observed, where the CO coverage reaches a CO molecule per step Pt atom, significantly higher than their close-packed analogous steps with straight terrace termination. For the O-saturated surface, a significantly higher O coverage is observed in kinked planes compared to the Pt(111) surface.
While the strong adsorption of CO at the kinked edges points toward a higher ignition temperature of the CO oxidation at kinks as compared to terraces, the large O coverage at steps may lead to an increased reactivity of kinked surfaces during the active stage of the CO oxidation.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Fernando García-Martínez, Elia Turco, Frederik Schiller, and J. Enrique Ortega (2024) CO and O2 Interaction with Kinked Pt Surfaces ACS Catal. doi: 10.1021/acscatal.4c00435 ↩