Autism and Neanderthal genome
Autism and Neanderthal genome
The evolution of the human being has a complex form in which some branches bifurcate and form two different species and some different branches come together again and form individuals with a certain degree of hybridization. The only hominin remaining today is Homo sapiens and the last to disappear was Homo neanderthalensis. Neanderthals, our closest relatives, spread throughout Europe and southwest Asia before their extinction approximately 30,000 years ago. Among the extinct species, Neanderthals are the closest hominins to us on the tree of life, the ones with the longest fossil record and the ones whose DNA we know best.
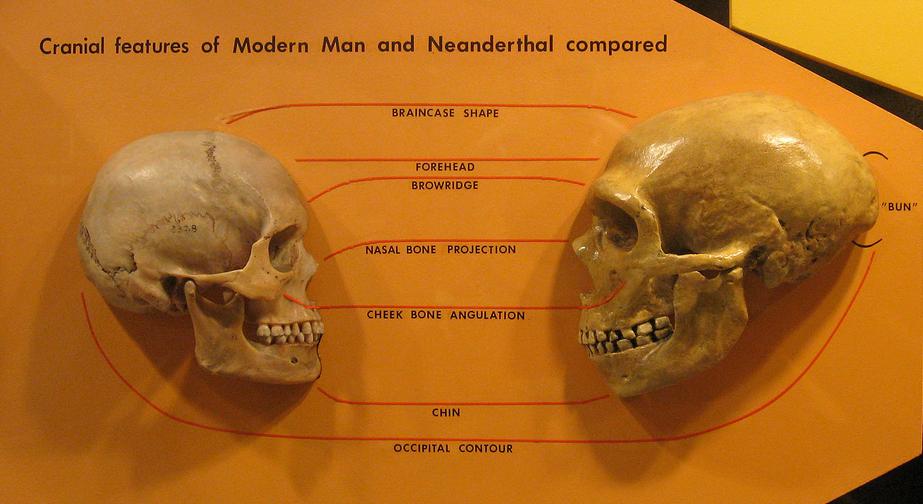
The year 2010 saw one of the discoveries of the century. Richard E. Green and 46 other scientists published an article in the journal Science whose last author was Svante Pääbo, Nobel laureate in 2022, in which they presented a draft sequence of the Neanderthal genome, created from three individuals, and compared it with the genomes of five modern humans 1. The genomic data suggested that Neanderthals interbred with the ancestors of modern humans and left traces of Neanderthal DNA in contemporary humans. In other words, the two species interbred and we are their descendants and therefore carry Neanderthal DNA mixed with Sapiens DNA in our cells.
As a result of these hybridizations, Eurasian-derived populations have approximately 2% Neanderthal DNA, which was acquired shortly after the sapiens ancestors migrated from Africa. These hybridization events occurred between 47 and 65 thousand years ago. Subsequently, a subset of Europeans migrated back to Africa approximately 20 thousand years ago, and carried some of this Neanderthal ancestry with them, so that all modern Africans have a small but measurable amount of Neanderthal DNA from this event.
The recent sequencing of more archaic human genomes has made it possible to ascertain the influence of alleles derived from these archaic humans on the health of the modern population. With respect to Neanderthal-derived variants, positive selection has been seen in genes related to immune function, skin and hair pigmentation, physiological responses to high-altitude conditions, aspects of metabolism, hypercoagulation and others. Scientists have also found associations between Neanderthal DNA and human conditions, including propensity for severe COVID, autoimmune diseases, prostate cancer, type 2 diabetes, hypercoagulation, pain sensitivity, depression and autism, but also some protective effects such as against symptoms of schizophrenia.
Most of the genes involved in brain development are highly conserved, if something is altered it is very likely that individual will not make it. On the other hand, there are strong links between some brain characteristics and Neanderthal DNA enrichment. Specifically, Neanderthal DNA enrichment is associated with less globularity of the skull, greater neural connectivity in the visual processing systems, particularly between the intraparietal sulcus and the occipital cortex and fusiform gyrus, and less connectivity within the default mode network, which acts when we are not focused on a task.
Many of these same connectivity patterns are observed in autism. For example, in autism, insufficient connectivity is observed in the default mode network while increased connectivity within visual processing networks is also a common feature in autism, reflected in the connectivity of occipital regions and frontal lobes. Autistics also often exhibit cognitive strengths in areas such as mathematics. Autistic children have greater activation in the fusiform gyrus and occipital lobes compared to non-autistic children, again suggesting that visual processing modalities are an area of strength in autism. Interestingly, many of these features are shared by nonclinical groups with high Neanderthal DNA content.
Pauly et al. 2 have discovered that individuals on the autism spectrum possess an enrichment of specific Neanderthal DNA types. Compared to ethnically matched control groups, the study revealed that autistic individuals tend to have more Neanderthal variants that are otherwise rare in the general population. This suggests, from a population perspective, that many of these variants are gradually disappearing from the human genome.
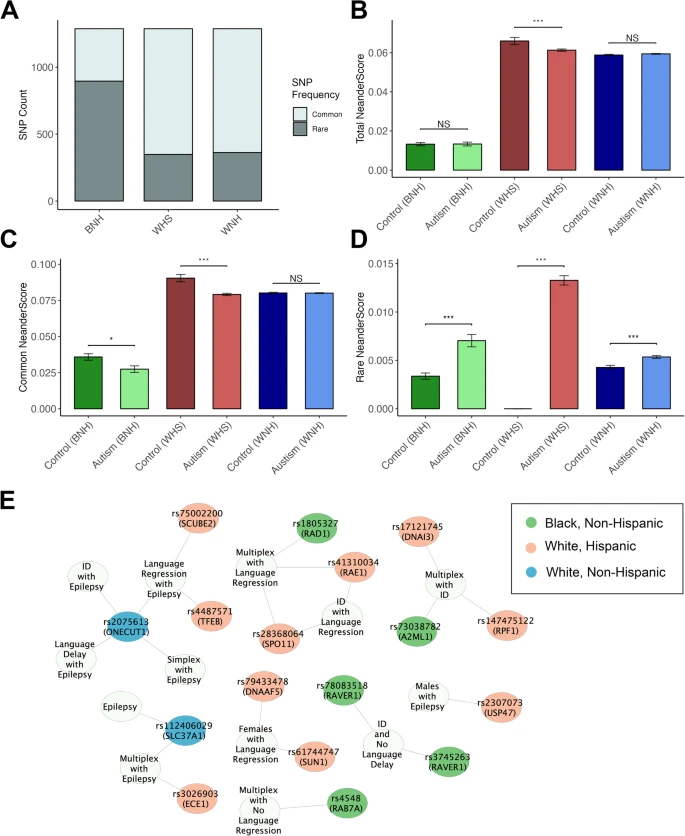
The researchers identified specific variants or polymorphisms associated with autism. By selecting only polymorphisms that influence gene expression in the brain (known as brain-associated quantitative trait loci or brain QTLs), they pinpointed 25 QTLs enriched in autism. One notable example is a common polymorphism in the SLC37A1 gene. Approximately 67% of non-Hispanic white autistics with epilepsy carried this variant, compared with 22% of controls and 26% of autistics without epilepsy. Although the functions of this gene are not well understood, it is expressed in the brain within a cellular organelle known as the endoplasmic reticulum, indicating it likely plays crucial roles in overall cellular metabolism. Interestingly, epilepsy often occurs in individuals with metabolic diseases.
Several authors have inferred the social organization of Neanderthals, reinforcing previous notions that they probably lived in small communities. This is suggestively related to the social difficulties of autistic individuals. Previous studies have found that the size of a primate species’ social group predicts neocortex size, and suggest links between group size and cognitive function. While Neanderthals appear to have maintained more localized patterns of social interaction, the early ancestors of modern European humans seemed to have engaged in larger and better-integrated social networks.
Despite these associations, the researchers emphasize that autistic individuals do not carry more Neanderthal DNA overall compared to non-autistic individuals. Instead, they possess a subset of Neanderthal-derived genetic variations that are more prevalent among people with autism and their families. Additionally, some genetic traits inherited from Neanderthals may increase susceptibility to autism.
Understanding the role of Neanderthal DNA in autism could provide new insights and approaches to diagnosis and enhance the understanding of autism’s diverse causes. One diagnostic example could involve using these markers in gene panels to aid in diagnosing autism.
References
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prüfer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Höber B, Höffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Ž, Gušic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, de la Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PLF, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Pääbo S (2010) A draft sequence of the Neanderthal genome. Science doi: 10.1126/science.1188021 ↩
- Pauly R, Johnson L, Feltus FA, Casanova EL (2024) Enrichment of a subset of Neanderthal polymorphisms in autistic probands and siblings. Mol Psychiatry. doi: 10.1038/s41380-024-02593-7. ↩