A new method to make valuable olefins from abundant carboxylic acids
A new method to make valuable olefins from abundant carboxylic acids

Organic chemistry is the science and the art of making carbon containing molecules. In these, carbon atoms may be linked by a single, double or triple bond to another carbon atom. Bonds are not more than the way we humans represent the sharing electrons between two atoms. In this regard, a single bond consists of two bonding electrons, a double bond of four and a triple of six. Single bonds are the most prevalent ones in organic molecules, but double bonds are of great importance, and they are present in fragrances, drugs and natural products. Moreover, they are especially interesting because contrary to single carbon-carbon bonds, which are typically inert, double bonds are reactive. This means that they can be transformed into other molecules easily, and the chemistry of double bonds (also called olefins) is very rich. Despite their importance, the methods for their synthesis are limited, and most of them rely on chemistry developed more than 30 years ago. Now a new method has been reported by the Baran Lab 1 which employs alkyl carboxylic acids, a ubiquitous and diverse building block (Figure 1).
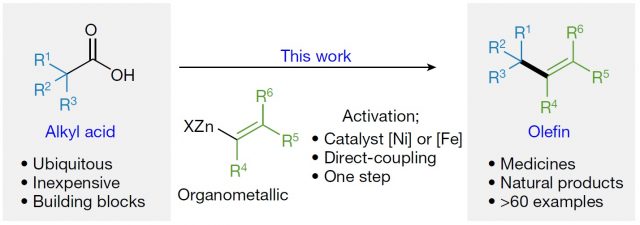
The protocol starts with the reaction of alkyl carboxylic acids with the commercially available tetrachloro-N-hydroxyphthalimide (TCNHPI), which results into redox-active esters (RAE). (Figure 2) These can be isolated (i.e., purified from other undesired products) but can also be used in situ. In any case, the next step is the formation of the double bond. This is achieved by coupling with an alkenylzinc reagent. These have to be prepared, but there are several robust methods already reported for that. The discovery of this research was that nickel(II) (and in some cases also iron) together with a simple and abundant ligand (2,2’-bipyridine) could catalyze the coupling thus affording the desired olefins (Figure 2).
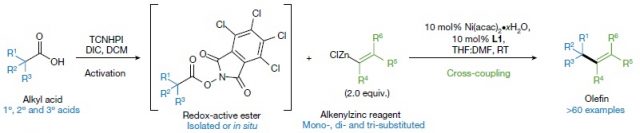
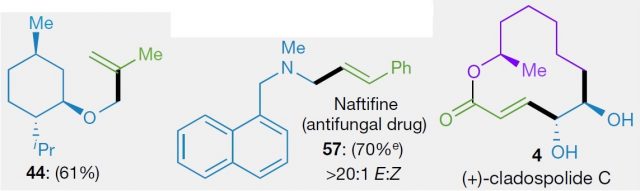
They have termed this new method decarboxylative alkenylation, and it is very self-explanatory. Alkenylation means the making of alkenes, which is just another term for olefins or double bonds. The decarboxylative term is because of the redox-active ester, upon accepting one electron leads to CO2 fragmentation and a radical formation. For example, the molecule between brackets in Figure 2 upon fragmentation would lead to the loss of the part drawn in black, with just the blue part remaining as a radical. This part is then coupled with the alkenylzinc reagent thanks to the nickel. Many different types of compounds could be made with this methodology, including medicines and natural products (Figure 3)
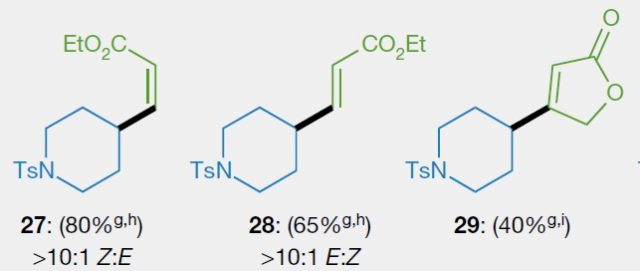
Apart from the abundance and low cost of carboxylic acids, another of the advantages of the new methodology is the control of stereochemistry. The stereochemistry of a molecule is how the different atoms are arranged in it. A double bond can have up to four substituents (groups at the ends) and their position can vary. This is illustrated in figure 4. Whereas there is only one option in the formation of compound 29, 27 and 28 have the CO2Et group in a different position. 27 has the two substituents(groups, blue and green) towards the same side, and in organic chemistry is termed Z. If they are pointing towards opposite direction as in 28 the stereoisomer is said to be E. These isomers are different molecules, and thus, their properties vary. Consequently, is important to be able to control the position of the substituents when double bonds are, but this is not straightforward. Currently, the method for the synthesis of an olefin is chosen according to which of the isomers is needed. The reaction mechanism of each method will determine which will be formed. Moreover, is common that with the existing methods a mixture of both isomers will be obtained. On the contrary, with this new method, the desired stereoisomer can be obtained, just the correct alkenylzinc reagent has to be prepared. Fortunately, this is not a big issue, as there are methods for accessing them.
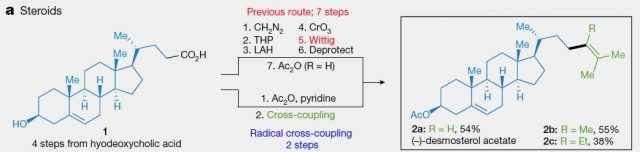
Another big advantage of this method is the step economy. In the article, it is showcased by the synthesis of several natural products, which needed fewer steps than with the traditional synthetic route. In figure 5 the synthesis of (-)-desmosterol acetate, a drug precursor, is shown. While the previous methodology required 7 steps to transform the steroidal substrate 1 into (-)-desmosterol acetate, the new protocol can furnish it in merely 2. The step economy results in higher yield too, and this is of crucial importance in the synthesis of drugs, as it makes their production cheaper. Moreover, this new methodology adds versatility and allows the easy synthesis of analogs (2b and 2c in Figure 5). Again, this is highly desirable for the finding of new drugs.
Double bonds are essential constituents of many natural products and drugs, but also important precursors for further chemistry, such as in polymer chemistry. The newly developed decarboxylative alkenylation turns abundant alkyl carboxylic acids into more difficult to make olefins with an operationally simple setup. This new discovery is likely to become soon a standard practice in organic synthesis.
References
- J. T. Edwards, R. R. Merchant, K. S. McClymont, K. W. Knouse, T. Qin, L. R. Malins, B. Vokits, S. A. Shaw, D. H. Bao, F. L. Wei, T. Zhou, M. D. Eastgate, P. S. Baran (2017) Decarboxylative alkenylation, Nature, 545, 213. DOI:10.1038/nature22307 ↩
1 comment
[…] La química de las olefinas es algo que nos rodea por todas partes (productos naturales, fármacos, perfumes, etc., etc.) y es de una importancia económica enorme. Sin embargo las fuentes y los métodos de obtención son muy del siglo […]