A new two-dimensional carbon allotrope combining graphene and nanoporous design
A new two-dimensional carbon allotrope combining graphene and nanoporous design
Carbon is one of the most versatile elements in the periodic table. Beyond the familiar forms of graphite and diamond lies a rich family of carbon structures with surprising and useful properties. Among these, graphene, a single two-dimensional (2D) allotrope consisting in a layer of carbon atoms arranged in a perfect hexagonal lattice, has captivated scientists and engineers because of its remarkable strength, electrical conductivity, and thinness. But graphene also has limitations: perfect graphene has no bandgap, meaning it behaves like a metal rather than a semiconductor. For many technologies, from transistors to chemical sensors, we need materials that conduct electricity in a more controllable way.
A new study reports 1 the creation of a previously unrealized 2D carbon allotrope that combines features of graphene with engineered holes (nanopores) and alternative carbon ring arrangements known as biphenylene segments. This structure bridges the gap between ideal graphene sheets and more complex functional materials, opening pathways toward applications in nanoelectronics and sensing technologies.
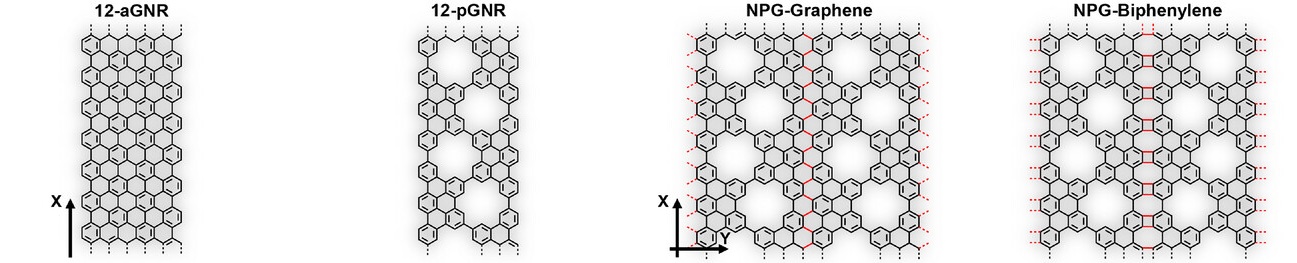
An allotrope with atomic precision
The researchers set out to combine nanoporous graphene with segments of biphenylene in a single, continuous 2D material. By carefully designing molecular precursors they were able to grow narrow porous graphene “nanoribbons” on a gold surface under ultra-high vacuum conditions. These nanoribbons had regularly spaced holes and specific edge structures. When heated under controlled conditions, neighboring ribbons fused laterally, forming an extended sheet with both graphene-like and biphenylene-like regions seamlessly connected.
What makes this achievement remarkable is the level of atomic precision the team could control. In conventional materials synthesis, defects and random structural variations often obscure the fundamental properties researchers want to study. But by building the material from the “bottom up” the authors ensured that the resulting structure was well-defined at the scale of individual atoms. The ability to deliberately include periodic pores and non-hexagonal ring structures allows tuning both the electronic properties of the material and its chemical reactivity.
A semiconducting bandgap
The presence of nanopores interrupts the otherwise continuous network of carbon atoms found in graphene. In graphene, electrons behave as if they have no mass near certain energies, which gives rise to its extreme conductivity but also means it cannot easily be switched “off” the way semiconductor devices need to be. Introducing a regular pattern of holes changes the electronic band structure of the material.
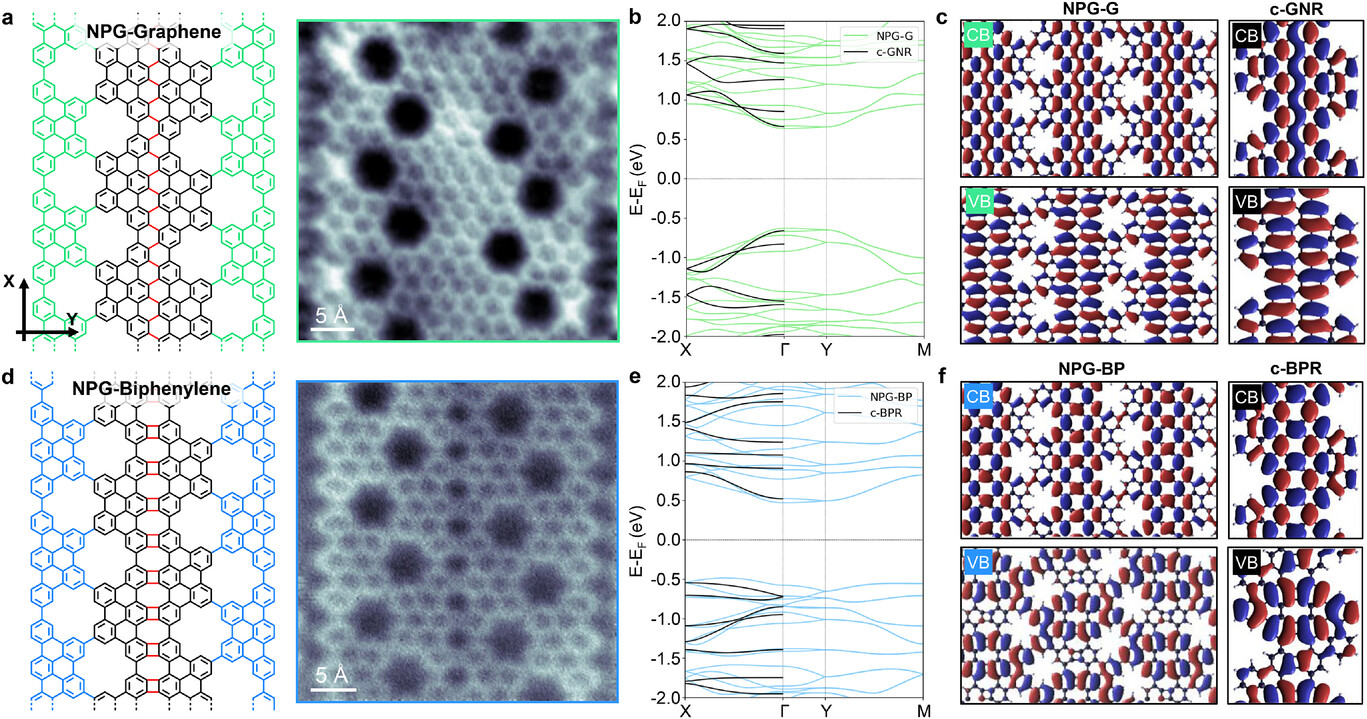
When the researchers characterized the material using scanning probe microscopy and theoretical calculations, they found that the resulting network exhibits a semiconducting bandgap. A bandgap is the energy difference between the highest occupied and lowest unoccupied electron states; having a non-zero bandgap is essential for transistor operation because it allows the material to switch between conducting and non-conducting states. Moreover, different structural motifs — graphene-like versus biphenylene-like — showed different bandgaps and behaviours, providing multiple ways to tailor the material’s properties.
Chemical functionality and stability
Another consequence of the design is the chemical functionality provided by the nanopores. These pores create regions that can interact more readily with molecules in the environment, making the material attractive for sensing applications. The authors note that the nanoporous structures showed a measurable affinity for small gas molecules like carbon monoxide. Materials that can selectively bind and detect certain molecules are of great interest for environmental monitoring, medical diagnostics, and industrial process control.
It is also worth noting the stability of this new carbon allotrope. Many functional 2D materials are sensitive to air or moisture, limiting their usefulness outside of controlled laboratory environments. In contrast, the synthesized nanoporous carbon networks were stable upon exposure to oxygen and air at ambient conditions. While further testing is needed to evaluate long-term durability, this initial robustness is promising for real-world applications.
A significant advance
This research demonstrates a significant advance in the synthesis of complex carbon materials. By combining regular nanopores with alternating graphene and biphenylene segments, the authors have created a new class of tunable 2D carbon material that bridges fundamental physics and practical function. The precise control over atomic structure enables modification of electronic behaviour while providing chemically active sites for interaction with the environment. These features suggest exciting potential for future technologies in electronics and sensing — and they exemplify how thoughtful design at the atomic level can open entirely new frontiers in materials science.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Paula Angulo-Portugal, Martin Irizar, Longfeng Huang, Mamun Sarker, Mustafa A. Ashoush, Zakaria M. Abd El-Fattah, Johannes Barth, Frederik Schiller, Afaf El-Sayed, Fei Gao, Dimas G. de Oteyza, Alexander Sinitskii, Aran Garcia-Lekue, Martina Corso, Ignacio Piquero-Zulaica (2025) A Functional 2D Carbon Allotrope Combining Nanoporous Graphene and Biphenylene Segments Advanced Materials doi: 10.1002/adma.202511706 ↩