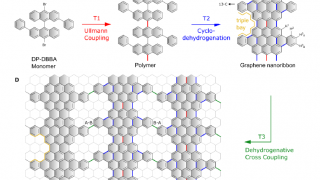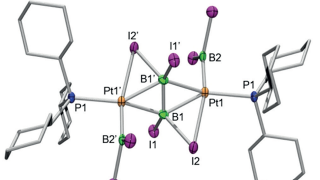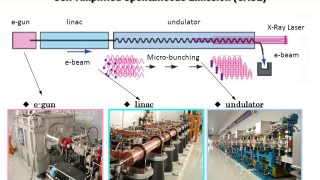
The multiscale nature of picocavities: a classical view to a quantum effect
Progress in nanotechnology has allowed controlling the morphology of metallic nanoparticles at the nanometer and even subnanometer scale, triggering the development of various applications in plasmonics and nanooptics, such as in enhanced vibrational spectroscopy, improvement of energy absorption of solar cells, optoelectronic circuits, quantum optics, nanosensing of biomolecules, or noninvasive thermotherapy in medicine. Most of […]