Riboflavin as a bioorthogonal photocatalyst
Riboflavin as a bioorthogonal photocatalyst
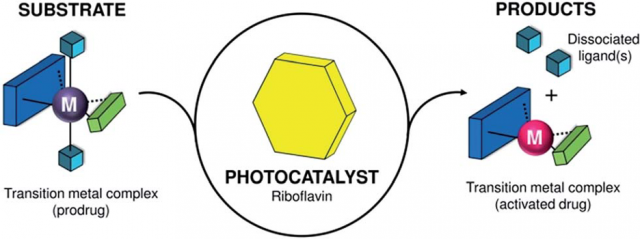
The combination of catalysis and bioorthogonality promises have an impact on drug discovery and bioimaging. Bioorthogonality, a term coined by Carolyn R. Bertozzi in 2003, refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. Hence, catalytic turnover can boost the efficiency of bioorthogonal chemical reactions, unveiling new strategies for prodrug activation and uncaging of molecular probes.
So far, transition metal and organometallic catalysts have opened new avenues for the advancement of bioorthogonal catalysis in cells. However, new biocompatible transformations are highly needed to further advance this extremely challenging field that is still in its infancy.
Metal complexes are typically regarded as catalysts that convert organic substrates into more valuable compounds; however, to date, catalytic transformations of metal complexes are practically unknown and represent a complete new way of thinking in catalysis. Their development can expand the scope of bioorthogonal chemical reactions to inorganic substances and metal-based prodrugs, fostering the creation of new inorganic chemistry toolkits for biology and medicine.
Now, a team of researchers from CIC biomaGUNE, UPV/EHU and DIPC describe 1 a new type of light-driven reaction in which the exogenous biological molecule riboflavin (Rf) functions as a bioorthogonal photocatalyst and a metal complex acts as an unconventional substrate.
This unusual catalyst/substrate pair relies on the photoredox properties of Rf to enable the selective activation of a PtIV prodrug (in Figure 2 below it is called ‘1’) of cisplatin with exceptionally low doses of blue light and induce apoptotic death in PC-3 human prostate cancer cells.
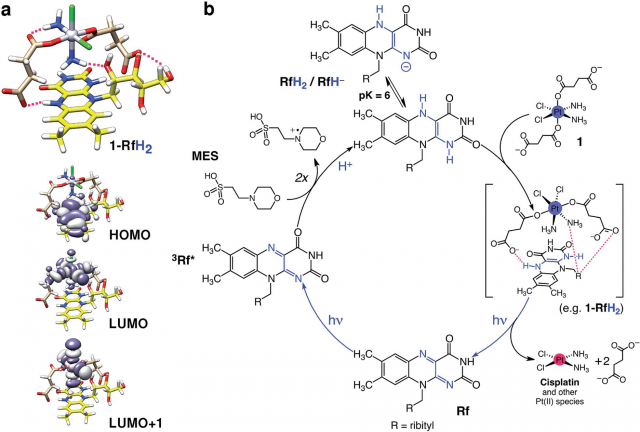
The co-administration of the Rf/1 as a catalyst/substrate couple enabled the photoconversion of 1 into a biologically active species by light irradiation at 460 nm, a wavelength that is ineffective when directly applied to the prodrug. In addition, the Rf/1 prodrug activation strategy induced an anticancer activity comparable to that of cisplatin with light doses up to 35 times lower than what is typically used for UVA and blue light activation of analogue platinum complexes.
In principle, photocatalysis can help expand the therapeutic potential of platinum prodrugs. Efficient light activation of PtIV complexes through catalysis may help localize the cytotoxic effects of Pt drugs, increase their dosing at the tumour target, and reduce their systemic toxicity. Rf (vitamin B2) is a highly biocompatible molecule, and its capacity to function in a bioorthogonal fashion may serve to enhance the selectivity of metal-based drugs by minimizing side reactions.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Silvia Alonso-de Castro, Emmanuel Ruggiero, Ane Ruiz-de-Angulo, Elixabete Rezabal, Juan C. Mareque-Rivas, Xabier Lopez, Fernando López-Gallego and Luca Salassa (2017) Riboflavin as a bioorthogonal photocatalyst for the activation of a PtIV prodrug Chemical Science doi: 10.1039/c7sc01109a ↩
1 comment
This is such an interesting article about riboflavin. Chemistry is awesome