Highly efficient, durable, and economically competitive hydrogen evolution electrocatalyst
Highly efficient, durable, and economically competitive hydrogen evolution electrocatalyst
Global energy demand is expected to rise around 30% by 2040 according to the International Energy Agency (IEA). Hydrogen (H2) produced by the electrolysis of water, using renewable electricity, the so-called green hydrogen, has emerged as a promising energy vector to respond to this increasing energy demand with the potential to decarbonize transportation, heating, and fine chemicals sectors. However, current technologies used to split water use expensive catalysts in extreme pH conditions that involve environmental pollution risks and handling hazards.
A hydrogen evolution reaction (HER) is any chemical reaction that yields H2. The conversion of protons to H2 requires reducing equivalents and usually a catalyst. In nature, HER is catalysed by hydrogenase enzymes. Commercial electrolysers typically employ platinum metal (Pt), which remains the state-of-the-art catalyst. However, Pt-based cathodes, besides being quite expensive, are only operative in acidic media. Thus, if we are to scale-up the HER toward the gigawatt-scale, we need highly efficient, durable and cheap electrocatalysts produced from earth-abundant materials, in addition to environment-friendly manufacturing techniques, including being operative under mild pH conditions.
Efficiency is related to the concept of overpotential, the potential that must be applied in an electrolytic cell in addition to the theoretical potential required to liberate a given substance at an electrode. Hence, the smaller the overpotential, the better. Copper sulphides (like Cu2S) and copper oxide present remarkably low overpotentials. On top of that, these compounds are stable compared to other transition metal catalysts.
However, these Cu2S electrocatalysts are measured under extreme pH conditions, i.e., highly acidic or alkaline electrolytes, thus increasing the maintenance costs and the potential risks of environmental pollution when scaling-up H2 production. Scarce studies of efficient HER on Cu2S electrodes in close to neutral conditions are reported.
On the other hand, scaling-up green H2 production does not only require cheap efficient electrodes but also durable ones. Continuous operation reported for Cu-based electrocatalysts ranges from a few hours up to several days. It is therefore apparent that the development of platinum-group-metal-free, low-cost, and highly efficient HER electrocatalysts still remains a challenge.
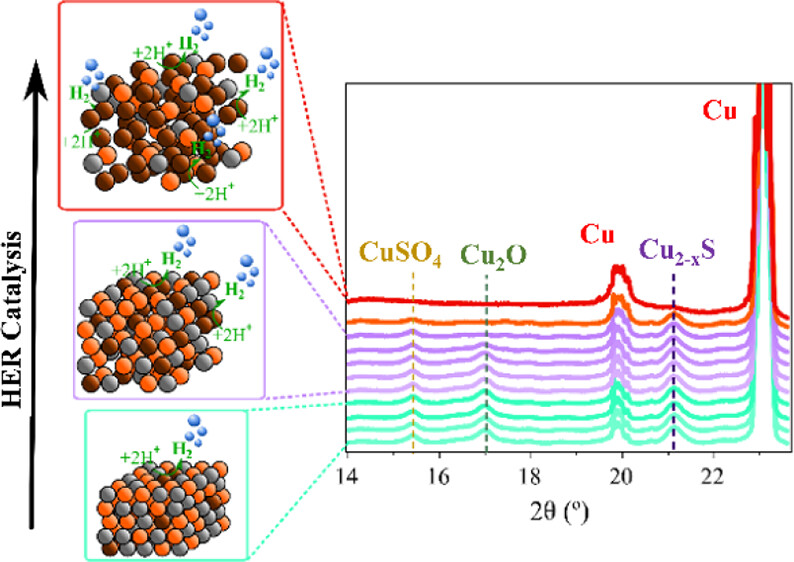
Now, a team of researchers reports 1 a cost-effective, facile method to synthetize a Cu2–xS electrocatalyst with overpotential values under mild conditions (pH 8.6) comparable to the lowest reported overpotential for Cu2–xS-derived catalysts operating at extreme pH. Importantly, the new Cu2–xS electrocatalyst exhibits a remarkable durability of almost 1 month, the longest reported to date for this material at high current densities. This new catalyst sets a technical breakthrough and a great step toward scalability for industrial renewable electrocatalytic hydrogen production.
The researchers carried out a comprehensive structural and chemical characterization of the electrodes before, during, and after the HER test, taking advantage of the combination of operando spectroelectrochemistry (SEC), X-ray diffraction (XRD), and X-ray photoemission spectroscopy (XPS). Analysis of Cu2–xS electrodes simultaneously to the electrochemical tests unveiled for the first time that the catalytic activity is mainly driven by catalytic centres located at the Cu species at the surface.
In summary, a fast, environmentally friendly, and cost-effective synthetic strategy, combined with the excellent performance in almost neutral pH conditions may pave the way for the future design of highly efficient, durable, and economically competitive hydrogen evolution electrocatalysts to be used in a wide range of electrosynthetic applications.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Roser Fernández-Climent, Jesús Redondo, Miguel García-Tecedor, Maria Chiara Spadaro, Junnan Li, Daniel Chartrand, Frederik Schiller, Jhon Pazos, Mikel F. Hurtado, Victor de la Peña O’Shea, Nikolay Kornienko, Jordi Arbiol, Sara Barja, Camilo A. Mesa, and Sixto Giménez (2023) Highly Durable Nanoporous Cu2–xS Films for Efficient Hydrogen Evolution Electrocatalysis under Mild pH Conditions ACS Catal. Doi: 10.1021/acscatal.3c01673 ↩
1 comment
[…] Researchers at the University of Toronto are using artificial intelligence to accelerate scientific breakthroughs in the search for sustainable energy. They have used the Canadian Light Source (CLS) at the University of Saskatchewan (USask) to confirm that an AI-generated […]